In this issue of Blood, Aljitawi et al demonstrate for the first time that erythropoietin (EPO) signaling directly inhibits hematopoietic stem and progenitor cell (HSPC) migration and that an acute and transient reduction in peripheral EPO levels, via hyperbaric oxygen (HBO) therapy just before transplant, is safe and can enhance hematopoietic engraftment in patients undergoing umbilical cord blood transplantation (UCBT).1
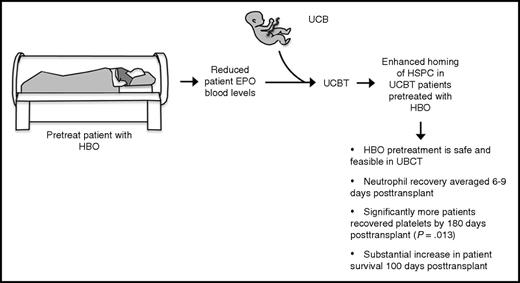
HBO pretreatment of UCBT is safe and may improve engraftment. A pilot clinical study was performed, with the primary end point of assessing the safety of HBO pretreatment of UCBT and secondary end points of evaluating the rates and kinetics of neutrophil and platelet engraftment. HBO pretreatment of UCBT was found to be safe and feasible. Neutrophil recovery averaged only 6 and 9 days for patients subjected to myeloablative and reduced-intensity conditioning, respectively, compared with 17 and 25 days for controls. One hundred percent of patients displayed platelet recovery by 180 days posttransplant, compared with only 69% of controls (P = .013). One hundred percent of HBO-treated patients survived 100 days posttransplant compared with only 76% of controls (P = .051).
HBO pretreatment of UCBT is safe and may improve engraftment. A pilot clinical study was performed, with the primary end point of assessing the safety of HBO pretreatment of UCBT and secondary end points of evaluating the rates and kinetics of neutrophil and platelet engraftment. HBO pretreatment of UCBT was found to be safe and feasible. Neutrophil recovery averaged only 6 and 9 days for patients subjected to myeloablative and reduced-intensity conditioning, respectively, compared with 17 and 25 days for controls. One hundred percent of patients displayed platelet recovery by 180 days posttransplant, compared with only 69% of controls (P = .013). One hundred percent of HBO-treated patients survived 100 days posttransplant compared with only 76% of controls (P = .051).
Since the first UCBT was performed successfully nearly 30 years ago to treat a patient with Fanconi anemia,2 umbilical cord blood (UCB) has provided a rich source of HSPCs, in addition to bone marrow and mobilized peripheral blood (mPB), for treating hematologic disease via transplantation. UCB is an appealing transplant source for several important reasons. First, it is widely and readily available. There are currently >700 000 units of cord blood cryopreserved worldwide.3 It is well documented that UCB can be frozen for long periods of time with no overt loss of viability or efficacy.4 Second, because the national bone marrow donor registries overrepresent donors of European descent, UCB provides an important alternative donor source, easing the difficulty of identifying unrelated allogeneic donors for patients from underrepresented ethnic groups. Finally, UCBT typically results in fewer immunological complications related to acute and chronic graft-versus-host disease than mPB or bone marrow.5
UCBT also presents significant challenges, however, that prevent it from being adopted as the first choice among clinicians for allogeneic transplant. Engraftment, as measured by neutrophil and platelet recovery, is significantly delayed in UCBT compared with mPB or bone marrow transplantation, which can expose patients to additional risk of infection and other complications.4 The delayed engraftment observed in UCBT most likely results from the limited number of cells available in the graft. Although >30 000 UCBTs have been performed since 1988, the vast majority of these patients were children, who require a delivery of fewer total HSPCs. Interestingly, more recent evidence suggests that UCB may also home less efficiently to the bone marrow niche relative to mPB and bone marrow.3
Tremendous effort has been exerted over the past several decades to overcome these barriers to the adoption of UCBT, especially in treating adults. Transplantation of multiple cord blood units into adults has provided only limited improvement, as 1 cord typically achieves dominance upon long-term engraftment.3,4 Most effort has focused on inducing the expansion of transplantable HSPCs ex vivo via optimization of cytokine cocktails, coculture with bone marrow stroma-derived cells, copper chelation, nicotinamide treatment, or treatment with expansion-inducing small molecules.3,4 Indeed, Wagner et al recently reported the results of a phase 1/2 clinical trial using SR-1, a small molecule antagonist of the aryl hydrocarbon receptor, to expand UCB ex vivo prior to transplant.6
Here, Hal Broxmeyer (an early pioneer and advocate of UCB transplantation) and colleagues tackle the limitations of UCBT by focusing on improving the homing efficiency of UCB HSPCs.1 They report the novel discovery that signaling via the EPO receptor on human and mouse HSPCs inhibits their migratory response to SDF-1, a master regulator of HSPC migration and bone marrow homing during transplant.7 They further demonstrate that blocking EPO or the EPO receptor via antibodies or short hairpin RNAs boosts HSPC migration. As HBO treatment of patients is known to reduce blood EPO levels and this group had previously shown that HBO treatment improves UCB HSPC engraftment in mice,8,9 they asked whether reduced EPO was responsible for enhanced engraftment in this model. Indeed, EPO treatment significantly ameliorated the engraftment benefit seen in mice after HBO therapy. This work is the first to demonstrate that EPO signaling regulates HSPC migration in response to SDF-1, revealing a novel axis of regulation.
These preclinical findings emboldened the authors to perform a pilot clinical study, with the primary end point of assessing the safety of HBO pretreatment of UCBT and secondary end points of evaluating the rates and kinetics of neutrophil and platelet engraftment (see figure). Here, the authors demonstrate that HBO pretreatment of UCBT is safe and feasible, with no major HBO treatment–related side effects observed. Further, their results compared favorably to previous controls with respect to several important measures. Neutrophil recovery averaged only 6 and 9 days for patients subjected to myeloablative and reduced-intensity conditioning, respectively, compared with 17 and 25 days for controls. One hundred percent of patients displayed platelet recovery by 180 days posttransplant, compared with only 69% of controls (P = .013), although the median time to platelet recovery was not notably different between treatment and control groups. Finally, 100% of HBO-treated patients survived 100 days posttransplant compared with only 76% of controls (P = .051).
This work by Aljitawi et al is highly significant because it demonstrates that a minimally invasive procedure (HBO pretreatment), which requires no manipulation of the actual cells being transplanted, has the potential to substantially improve clinical outcomes in UCBT and overcome some of the barriers to making this a viable procedure for many patients. Although some of the clinical data failed to meet a bar of statistical significance, a prospective randomized study seems warranted as the logical next step.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal