In this issue of Blood, Basiorka et al have challenged how we think about cell death in myelodysplastic syndromes (MDS), proposed an explanation for the shared morphologic changes that are seen despite genetic heterogeneity, and identified a new target for therapeutic intervention.1
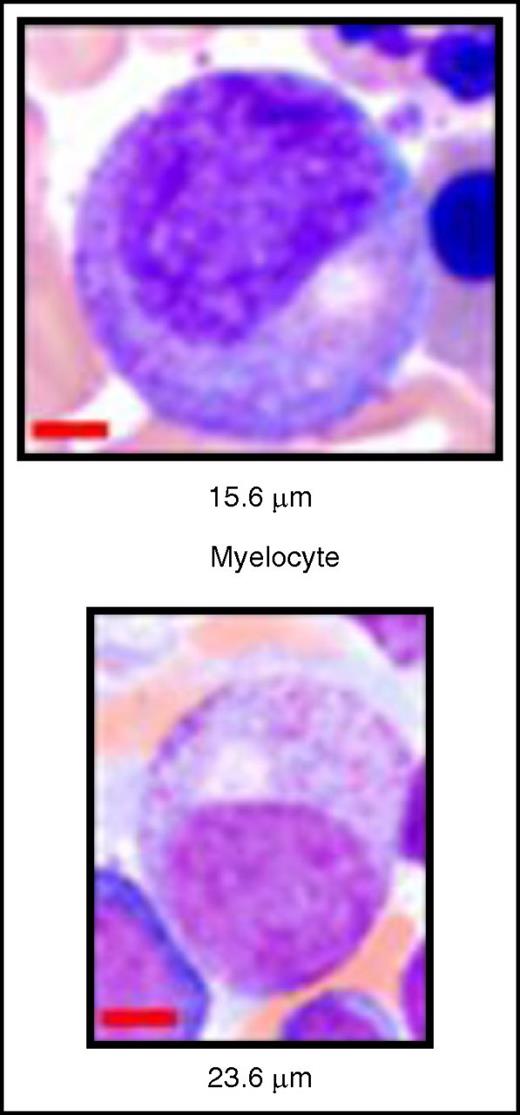
Normal vs increased cell diameter in a dysplastic myelocyte. (Top) Normal myelocyte. (Bottom) Enlarged dysplastic myelocyte with mild hypogranulation. See Figure 3G-H in the article by Basiorka et al that begins on page 2960.
Normal vs increased cell diameter in a dysplastic myelocyte. (Top) Normal myelocyte. (Bottom) Enlarged dysplastic myelocyte with mild hypogranulation. See Figure 3G-H in the article by Basiorka et al that begins on page 2960.
The contradiction of peripheral cytopenias despite normal or even hypercellular bone marrow in patients with MDS has been attributed to excessive apoptosis of early marrow progenitors despite high fractions of proliferating cells.2 In addition to morphologic bone marrow examination, early studies utilizing techniques such as terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling and in situ end labeling of fragmented DNA confirmed an increase in the percentage of myeloid, erythroid, and megakaryocytic cells undergoing apoptosis, although this did not appear to correlate with the degree of cytopenias.3,4 More recently, flow cytometric detection of annexin V binding has confirmed increases in CD34+ cell apoptosis in refractory anemia/refractory anemia with ringed sideroblasts and refractory anemia with excess blasts, whereas disease progression was associated with a reduction in apoptosis, likely reflecting differences in activation of the extrinsic and intrinsic pathways of apoptosis, respectively, over the course of the disease.5,6 The concept of apoptosis leading to ineffective hematopoiesis has been foundational in our understanding of this disease. What if this is not the whole story?
There are additional mechanisms of cell death that include autophagy and pyroptosis, which is also known as caspase 1–dependent programmed cell death.7,8 Caspase 1 is not involved in apoptosis, and in fact its activity results in cell death that is distinct from that seen in apoptosis mediated by other caspases, such as caspase 3, 6, or 8.7 Nod-like receptors (NLRs) are upstream of caspase 1 and when stimulated bind an adaptor protein apoptosis-associated speck-like protein, forming a multiprotein complex termed the inflammasome which contains a caspase 1 activation and recruitment domain.7 Once caspase 1 is activated by the inflammasome, pore formation occurs within the plasma membrane of the cell, creating ionic gradients that then lead to water influx, cell swelling, and ultimately cell lysis.7 Importantly, caspase 1 has another key function as it catalyzes the conversion of precursors of the inflammatory cytokines interleukin-1β (IL-1β) and IL-18 into their active forms.7 The duality of function of caspase 1 is significant as it ties inflammation to cell death via a common intermediary, a finding that raises additional questions. Does pyroptosis significantly contribute to cell death in MDS? Could this explain other more puzzling observations, such as the inflammatory cellular environment and shared morphologic phenotypes despite chromosomal and mutational heterogeneity?9
In the current issue Basiorka et al asks these questions and through a series of elegant in vitro and in vivo experiments, carefully dissects the role of the nod-like receptor NLRP3 as part of the inflammasome complex that drives pyroptotic cell death in MDS hematopoietic stem/progenitor cells.1 There are several findings to note. First, as compared with age-matched controls, activation of the NLRP3 inflammasome complex was greater regardless of International Prognostic Scoring System risk or genotype, and there was a significant increase in the percentage of pyroptotic cells as compared with apoptotic cells in MDS patient samples. In addition, increased levels of S100A9, a damage-associated molecular pattern that is released during inflammation or cell death, were noted in the bone marrow plasma of low- and intermediate-risk MDS samples and found to be capable of activating NLRP3 and thus inducing inflammasome assembly. S100A8/9 heterodimers also activate NADPH oxidase, creating reactive oxygen species (ROS) that are then able to stimulate the NLRP3 inflammasome and drive pyroptosis. Interestingly, somatic gene mutations in MDS can lead to ROS generation,10 and indeed, Basiorka et al show that ROS activate β-catenin, which then initiates pyroptosis within cells with U2AF1 and SF3B1 splicing mutations. As mentioned previously, pyroptosis leads to the creation of plasma membrane cation channels that lead to cell swelling. From a morphologic standpoint, MDS bone marrow mononuclear cells had a larger mean cell area compared with controls. The authors propose that this process explains the larger cell size observed in MDS (see figure).
Finally, although an understanding of these mechanisms is relevant because they define disease pathogenesis, the authors importantly investigated how the inflammasome complex might be targeted and potentially lead to novel therapies for patients, something that is unquestionably needed. In an S100A9-transgenic mouse model that recapitulates MDS, ICTA, an icariin derivative, inhibited NLRP3 inflammasome activation and led to improvements in hemoglobin, leukocyte, and platelet cell counts. In addition, direct inhibition of NADPH oxidase reduced NLRP3 inflammasome assembly.
At times it can be challenging to envision how preclinical in vitro and animal model data might directly and immediately translate into information that clinicians can use. However, Basiorka and colleagues have been able to make their findings relevant both to those who care for patients with this disease and for those who teach trainees about MDS. Pyroptosis will now need to be considered as not only a significant contributor to cell death in MDS but also as an etiology for the morphologic changes that we observe and as a future pharmacologic target.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal