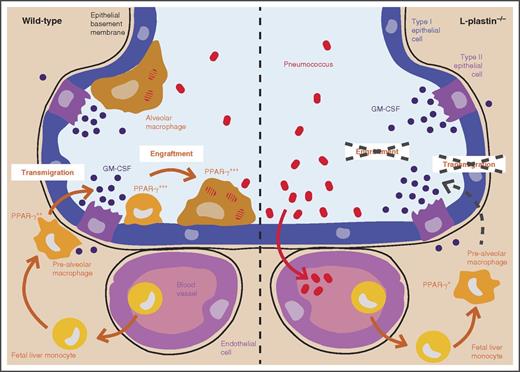
In this issue of Blood, Todd et al demonstrate that L-plastin (LPL) is crucial for the neonatal transmigration and engraftment of macrophage precursors into the alveolar space.1
Fetal liver monocytes seed the lungs between embryonic day 14.5 and birth.2 These cells then differentiate into prealveolar macrophages and acquire PPAR-γ expression through GM-CSFR signaling.3 These prealveolar macrophages then translocate and engraft into the alveolar space in the first week of life, where they develop into terminally differentiated alveolar macrophages. In LPL-deficient mice, the translocation and the engraftment into the alveolar space are impaired (compare left and right panel), resulting in a severe reduction in number of alveolar macrophages and in an increased susceptibility to pneumococcal infection.
Fetal liver monocytes seed the lungs between embryonic day 14.5 and birth.2 These cells then differentiate into prealveolar macrophages and acquire PPAR-γ expression through GM-CSFR signaling.3 These prealveolar macrophages then translocate and engraft into the alveolar space in the first week of life, where they develop into terminally differentiated alveolar macrophages. In LPL-deficient mice, the translocation and the engraftment into the alveolar space are impaired (compare left and right panel), resulting in a severe reduction in number of alveolar macrophages and in an increased susceptibility to pneumococcal infection.
Most tissue-resident macrophages derive from embryonic precursors that seed the various organs before birth and then self-maintain throughout life. Alveolar macrophages develop primarily from fetal liver monocytes that colonize the embryonic lungs 1 week before birth, differentiate into prealveolar macrophages shortly before birth, and then transmigrate into the alveolar space and further develop into terminally differentiated alveolar macrophages within the first week of life.2 This developmental pathway is driven by granulocyte-macrophage colony-stimulating factor (GM-CSF), which induces the expression of the critically required transcription factor peroxisome proliferator-activated receptor-γ (PPAR-γ).3 The mechanisms involved in colonization of the alveolar macrophage niche by embryonic precursors remain poorly defined. Todd et al studied the role of the actin-bundling protein LPL in alveolar macrophages. LPL is part of the α-actinin family and is widely expressed in immune cells (see www.immgen.org), playing an important role in T-cell motility,4 neutrophil activation,5 and marginal zone B-cell development.6 To study the function of LPL in alveolar macrophages, the authors crossed CD11c.CRE mice onto LPL-floxed mice, yielding mice with a dramatic reduction in the number of alveolar macrophages.
Although LPL RNA is highly expressed throughout the distinct stages of alveolar macrophage development pathway (M.G., unpublished results), the fact that CD11c.CRE × LPL-floxed mice have an important alveolar macrophage defect suggests that LPL is required after the prealveolar macrophage stage, the stage at which CD11c is first expressed.2 Moreover, equal numbers of fetal monocytes and pre–alveolar macrophages were found in wild-type and LPL−/− mice. In the first week of life, only a twofold reduction in alveolar macrophages is observed in LPL−/− mice as compared with wild-type mice, but in adults there is a 10-fold reduction. The authors investigated multiple potential mechanisms that might explain this loss of alveolar macrophages in LPL−/− mice.
LPL−/− macrophages had lower PPAR-γ expression. The lower PPAR-γ expression was not due to impaired production of GM-CSF by the lung epithelial cells, because the levels of GM-CSF found in the lungs of LPL−/− mice were not decreased compared with wild-type mice and were even slightly higher, which may suggest diminished consumption of GM-CSF. The lower PPAR-γ expression was also not due to impaired expression of the granulocyte-macrophage colony-stimulating factor receptor (GM-CSFR) or to deficiency of GM-CSFR signaling, as prealveolar macrophages and macrophages from wild-type and LPL−/− mice displayed similar GM-CSFR signaling (measured by STAT-5 phosphorylation). Moreover, treatment of LPL−/− alveolar macrophages with GM-CSF ex vivo induced normal PPAR-γ expression. Because the GM-CSFR and PPAR-γ responsiveness seemed normal, the authors next wondered whether monocyte localization in the lungs was compromised in the absence of LPL. The authors hypothesized that alveolar macrophage precursors need to localize in close contact with the lung epithelial cells that are the main source of GM-CSF in the lungs to undergo alveolar macrophage development and therefore evaluated whether LPL was required for monocyte trafficking and engraftment into the alveolar space. First, they demonstrated that IV-transferred LPL−/− monocytes failed to transmigrate into the alveolar space upon intratracheal instillation of CCL2. Next, they demonstrated that in LPL−/− mice there was a lower percentage of CD11c+ cells found within the alveolar space as compared with wild-type mice, supporting a role for LPL in transmigration into the alveolar space (see figure). In addition, they also found that when LPL−/− and wild-type monocytes were transferred into the lungs of neonatal mice, LPL−/− monocytes had a much lower capacity to engraft into the lungs. A similar effect was observed when LPL−/− and wild-type alveolar macrophages were transferred, demonstrating that LPL remains important for retention of alveolar macrophages into the alveolar space. Finally, the authors demonstrated that alveolar macrophages from LPL−/− mice displayed less podosomes, which are actin-rich structures required for migration and adhesion of macrophages. The diminished number of podosomes is therefore likely the reason LPL−/− monocytes fail to transmigrate and engraft into the alveolar space. Whether other tissue-resident macrophages also require LPL for their engraftment is unknown, but the authors recently reported that peritoneal macrophages are much less affected by loss of LPL than alveolar macrophages, suggesting that LPL is not as critical for all macrophages.7
These findings shed light on the function of LPL in alveolar macrophage development, but are also clinically relevant because the authors demonstrated that mice lacking LPL in alveolar macrophages in CD11c.CRE × LPL-floxed mice were much more susceptible to pneumococcal infection. This increased susceptibility is most likely due to the reduced numbers of alveolar macrophages in these mice because it has been shown that alveolar macrophages can clear up to 109 bacteria before there is significant uptake by lung dendritic cells.8 Although the authors showed that the number of CD11c+ dendritic cells and CD11c+ eosinophils was not affected in CD11c.CRE × LPL-floxed mice, it is impossible to rule out that dendritic cells or eosinophils are functionally impaired at this stage. Moreover, pneumococcal infection will recruit monocytes that will differentiate into inflammatory monocyte-derived cells, and these cells often express CD11c. The best way to demonstrate that the susceptibility of CD11c.CRE × LPL-floxed mice is due to the lack of alveolar macrophages would be to transfer wild-type monocytes in the lungs of CD11c.CRE × LPL-floxed mice to generate a wild-type population of alveolar macrophages in these mice and subsequently evaluate whether the resistance to pneumococcal infection is restored. Such a transfer strategy was recently applied in an influenza infection model.9 It remains to be seen whether patients carrying LPL mutations are also more susceptible to pulmonary infections. If so and if lack of alveolar macrophages is indeed the main reason for increased susceptibility, then transfer of wild-type monocytes into the lungs of LPL-deficient patients may yield long-tem protection because it has now been demonstrated that alveolar macrophages also have the capacity to self-maintain in the human lungs for several years.10
Conflict-of-interest disclosure: The authors declare no competing financial interests.