Abstract
Background:
Survivors of childhood Hodgkin lymphoma (HL) have an increased risk of subsequent malignant neoplasms (SMN). Response-adapted treatment for HL may decrease the SMN risk by reducing therapy in patients who have a good initial response to treatment. The Children's Oncology Group Study AHOD0031, which enrolled patients from 2002 - 2009, evaluated the role of early treatment response in directing therapy for children and adolescents with intermediate-risk Hodgkin lymphoma. We report the incidence and risk factors for SMN among 1,711 eligible patients enrolled on AHOD0031.
Methods:
Eligible patients had clinical stage I-IIA with bulk, I-IIAE, I-II B, IIIA-IVA with or without bulk. Patients initially received 2 cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone and cyclophosphamide (ABVE-PC), followed by a response evaluation of rapid early (RER) versus slow early responder (SER) status. All SER patients were randomized to an additional two cycles of ABVE-PC +/- 2 cycles of dexamethasone, etoposide, cisplatin and cytarabine (DECA). All SER patients received 21 Gy involved field radiotherapy (IFRT) following completion of chemotherapy. RER patients received 2 additional cycles of ABVE-PC. RER with complete responder (CR) status (RER/CR) patients were randomized to involved field radiotherapy (IFRT) or no further therapy. RER/non-CR patients were non-randomly assigned to IFRT. At a median follow-up of 6.2 years, an analysis of SMN was undertaken. The cumulative incidence of SMNs as a first event was calculated from the time of diagnosis adjusted for competing events due to relapse or death. Comparisons of the cumulative incidence among treatment groups were made using the K-sample test. A multivariable analysis using the Cox proportional hazard model assessed risk conferred by age, gender, race, disease stage, B symptoms, bulk disease, and IFRT.
Results:
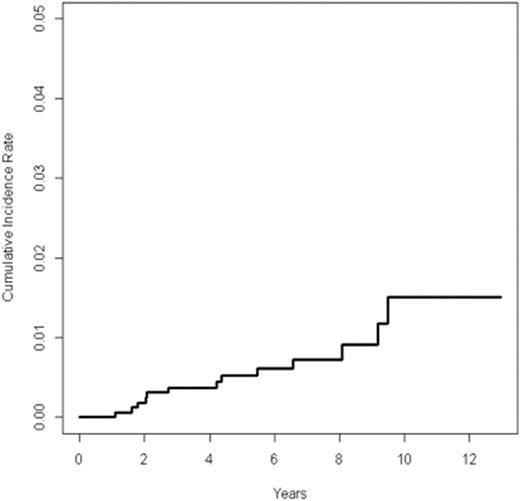
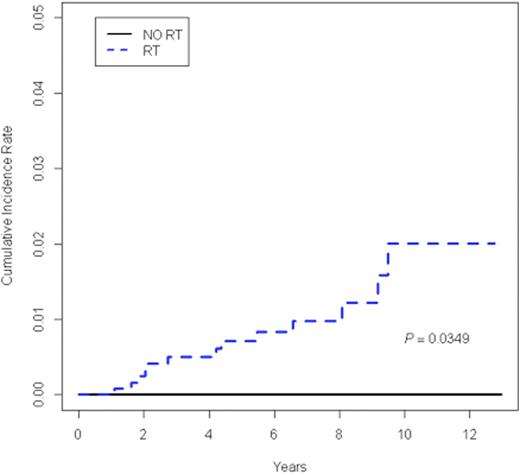
Among 1,711 patients with a median follow up of 6.2 years, excluding non-melanoma skin cancer, 16 SMNs occurred, 13 as a first event. SMNs included seven hematologic malignancies and nine solid tumors. No subsequent breast cancer has been reported to date. Patients who experienced a SMN did not differ from those who did not with respect to age, gender, race, disease stage, B symptoms, or bulk disease. The median time to SMN as a first event was 4.1 years (range 1.1-9.5 years). The 5-year cumulative incidence of SMN as a first event was 0.53% (95% CI 0.16 to 0.90) (Figure 1A). A comparison of the cumulative incidence of SMN in patients who received IFRT and those who did not demonstrated an increased incidence of SMN in the IFRT group (p = 0.03) (Figure 1B). In fact, to date, all SMNs have occurred in patients who received IFRT. Therefore, a multivariate analysis was conducted among patients who received IFRT, which included age, gender, race, disease stage, B symptoms, and bulk disease. The presence of B symptoms was independently associated with increased risk for SMN with a hazard ratio of 15.24 (95% CI 1.73-134.04, p=0.01). Among the 13 patients with a SMN as first event, there were 4 deaths.
Conclusions:
With a median follow up of 6.2 years, the 5-year cumulative incidence of SMN in patients treated with response-adapted treatment on AHOD0031 is very low. It is likely that we have captured the cases of secondary leukemia related to etoposide exposure, as these generally occur within five years of treatment. Longer follow-up will be needed to assess the risk of solid tumors, which are generally known to have longer latencies without plateau in incidence over time. Evaluation of the location of secondary solid tumors relative to the IFRT fields is in progress. As AHOD0031 demonstrated no benefit in event-free or overall survival for RER/CR patients treated with IFRT, future intermediate risk patients treated according to this treatment strategy will be less likely to receive IFRT and can be expected to be at lower risk of SMN.
Cumulative incidence of SMN among all patients treated on AHOD0031 (n=1711)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal