Abstract
PD-L1/PD-L2 are immunomodulatory molecules that engage with the PD-1 receptor on immune effector T and NK-cells to inhibit anti-lymphoma immunity. PD-1/PD-L1/PD-L2 axis molecules are prognostic in Hodgkin Lymphoma (HL, Roemer et al J Clin Oncol 2016) and Diffuse Large B-cell Lymphoma (DLBCL, Keane et al Lancet Haem 2015). Importantly, blockade of the axis is associated with particularly potent clinical responses in relapsed/refractory HL (Ansell et al NEJM 2015), as well as response in DLBCL (Armand et al J Clin Oncol 2013). Focus has been on the interaction of PD-L1 on malignant B-cells with PD-1 on HLA-class I restricted CD8+ effector T-cells. This is despite considerable evidence that: A) malignant B-cells in HL and DLBCL frequently lack the ability to present HLA-class I due to mutations in b2M and associated antigen presenting molecules (Challa-Malladi et al Cancer Cell 2013). This makes them insensitive to direct lysis by CD8+ T-cells (Zaretsky et al NEJM 2016) but potentially enhances their sensitivity to NK-cells; B) PD-L1/PD-L2 are expressed by inhibitory CD163+ monocytes/macrophages as well as by malignant B-cells (Chen et al CCR 2013). Here, we seek to establish the contribution of NK-cells and inhibitory CD163+ expressing monocytes/macrophages in the setting of HL and DLBCL.
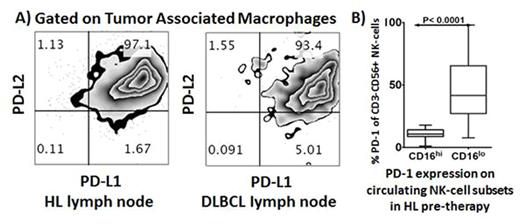
CD163/PD-1/PD-L1/PD-L2 gene expression was quantified by nanoString in 194 patients and was elevated in HL relative to DLBCL tissues (P<0.01, <0.01, <0.0001, <0.0001 respectively). By FACS, intratumoral tumor associated macrophages (TAMs) demonstrate pronounced protein expression of PD-L1/PD-L2 within HL and DLBCL diseased lymph nodes (Fig A). Pre-therapy blood was tested in 114 patients. Interestingly levels in each of total monocytes, CD14+HLA-DRlo monocytoid derived suppressor cells (moMDSC) and CD163+CD14+ monocytes were equivalent between lymphoma sub-types. However, consistent with tissue findings, there was marked increase in PD-L1 expression on CD14+ monocytes, moMDSC and CD163+CD14+ monocytes in HL compared to DLBCL patients (P<0.001, <0.0001 and 0.0086 respectively).
The NK-cell marker CD56 were higher in HL compared to DLBCL tissues (P<0.0001). Levels of PD-1 on circulating NK-cells were 7-fold elevated in HL relative to DLBCL (P<0.0001), whereas CD4+ and CD8+ T-cell PD-1 levels were equivalent between lymphoma sub-types. NK-cells can be subdivided into CD3-CD56dimCD16+ and CD3-CD56hiCD16- subsets. The CD16- subset produces abundant cytokines but are only weakly cytotoxic before activation. Although CD16- NK-cells are typically <10% of all NK-cells in the healthy circulation, we show their relative proportion is markedly expanded by 3.5-fold in HL patients. This is of particular importance since CD16- NK-cells are enriched in secondary lymphoid tissues, i.e. the context in which lymphoma resides. Notably, CD3-CD56hiCD16- NK-cells had substantially higher PD-1 expression relative to CD3-CD56dimCD16+ cells (P<0.0001, Fig B). A similarly aberrant NK-cell phenotype was observed in DLBCL.
An in-vitro functional model of TAM-like monocytes was developed to demonstrate the potential impact of inhibitory CD163+ expressing monocytes/macrophages on NK-cells in HL and DLBCL. Monocytes were cultured with the M6 TAM inducing cytokine cocktail of M-CSF and IL-6. Consistent with an inhibitory phenotype, M6 cultured monocytes were highly enriched for CD163 (P<0.001) and PD-L1 (P=0.0024). Critically, M6 cultured monocytes suppressed activation of primary NK-cells in direct cytotoxicity and ADCC assays against lymphoma targets. In line with these findings, depletion of circulating monocytes from the blood of pre-therapy HL and DLBCL patients enhanced NK-cell activation relative to monocyte intact PBMC, whereas this was not observed in age/gender-matched healthy control participants. Interestingly, the increase in NK-cell activation following monocyte depletion was most pronounced in the CD16-CD56hiCD3- NK-cell subset.
We describe a hitherto unrecognised immune evasion strategy mediated via skewing towards an exhausted PD-1 enriched CD16-CD56hiCD3- NK-cell phenotype. In addition to inhibition of NK-cells by the malignant B-cell, suppression of NK-cells occurs by PD-L1/PD-L2 expressing tumor associated macrophages. This mechanism is more prominent in HL than DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal