Abstract
Introduction
Cancer patients are at high risk for morbidity and mortality due to thrombosis and bleeding, in addition they may also have pre-existing conditions, such as atrial fibrillation (AF) and prior venous thromboembolism (VTE), for which chronic anticoagulation may be indicated. Historically, warfarin was the most commonly prescribed anticoagulant for stroke prevention in AF patients. Since 2010, the US FDA has approved 4 alternative direct oral anticoagulants (DOACs) - dabigatran, rivaroxaban, apixaban, edoxaban to reduce the risk of stroke and systemic embolism in patients with AF. There is limited literature to support the use of DOACs in cancer patients, but they are being prescribed by medical professionals nonetheless. Therefore, additional research is necessary to determine the effectiveness of the DOACs in cancer patients, to quantify their bleeding risks, and to identify patients that are more likely to benefit from these new medications in comparison to warfarin. Large randomized clinical trials of DOACs compared to warfarin in cancer patients have not been performed. Our aim was to determine the effectiveness and associated risk of DOACs vs. warfarin in a large, real-world population of cancer patients with AF.
Methods
We identified 532,743 AF patients initiating oral anticoagulant use in 2010-2014 continuously enrolled in MarketScan databases. We selected 41,036 cancer patients with inpatient or outpatient ICD9 code 140.x-172.x and 174.x-209.x (excluded 173.x, non-melanoma skin cancer), and then identified a subset of cancer patients being actively treated at the time of anticoagulant initiation. Active cancer patients were defined by the use of chemotherapy, radiation therapy or cancer surgery within 6 months prior to the start of anticoagulation. Patients were categorized according to the first anticoagulant prescribed after AF diagnosis. DOAC users were matched with warfarin users by age (±3 years), sex, enrollment date (90 days), and anticoagulant initiation date (90 days). Study endpoints, including ischemic stroke, severe bleeding (intracranial hemorrhage, gastrointestinal bleeding), other bleeding (genitourinary bleeding, hemopericardium, hemarthrosis, epistaxis, hemoptysis and unspecified hemorrhage) and VTE were identified using inpatient diagnostic codes, and VTE was additionally identified from 2 outpatient diagnostic codes within the same year.
Cox proportional hazards models were used to access the association between type of anticoagulant and outcomes adjusting for high-dimensional propensity score, age, sex, CHA2DS2-VASc score, and the prevalent outcome.
Results
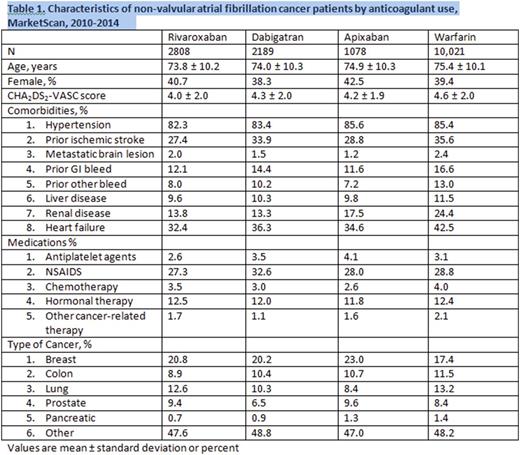
The demographic profile of the patients included in the analysis are depicted in Table 1, and Table 2 summarizes the outcomes data.
In this analysis there were 6,075 cancer patients with AF who were on DOACs (rivaroxaban 2808, dabigatran 2189, and apixaban 1078) compared to 10,021 on warfarin, with a mean age of 74 years, and a mean follow-up time of 1 year. Approximately 40% of the patients were woman, and breast cancer was the most common cancer in each cohort. We found that for rivaroxaban and dabigatran users, the adjusted hazard ratios for severe bleeding was non significantly different to warfarin users, however, apixaban users had a lower risk of severe bleeding compared to warfarin users, with a hazard ratio 0.37 (95% confidence interval 0.17-0.79, p=0.01). The risk of other bleeding was lower in dabigatran users compared to warfarin users with a hazard ratio 0.58 (95% confidence interval 0.41-0.84, p=0.003). The risk of ischemic stroke did not differ significantly amongst different anticoagulant users. Each of the DOACs was superior to warfarin in lowering the risk of incident VTE, with p values < 0.0001.
Conclusion
Based on this analysis the DOACs seem to be well tolerated in cancer patients in regard to the management of atrial fibrillation, with lower or similar rates of bleeding and stroke compared to warfarin users. Importantly, the DOAC users had a significantly lower risk of incident VTE. Given that VTE events contribute to significant morbidity and mortality in cancer patients, prescription of DOACs in place of warfarin can be considered in cancer patients with AF, while we are awaiting prospective data from randomized trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal