Abstract
Background: Hemophilia carriers report abnormal bleeding, even when factor VIII or IX levels are normal. Information comparing bleeding events between carriers and women with other inherited bleeding disorders is lacking.
Purpose: The purpose of our study was to characterize bleeding in hemophilia carriers using the International Society on Thrombosis and Hemostasis Bleeding Assessment Tool (ISTH-BAT) and to compare it with bleeding in normal controls, women with Type 1 VWD and Type 3 VWD obligate carriers (OC).
Method: This was a prospective, observational, cross-sectional study performed by members of GEHEP (Global Emerging HEmostasis Panel). Study participants were recruited from GEHEP members' clinics in North America (Kingston, Canada, Detroit and Philadelphia, USA), Europe (Malaga, Spain; Milan, Italy; Munich, Germany; Oslo, Norway) and South Africa (Johannesburg). Potential participants were identified through local patient databases and approached during clinic visits. All participants signed informed consent. Hemophilia carriers were defined by a documented FVIII or FIX mutation and/or by an appropriate family history (daughter of a man with hemophilia or mother of two sons with hemophilia or mother of one son with hemophilia with at least one other affected male relative). Demographic information was collected using a CRF and the ISTH-BAT was completed for each participant by study personnel. Existing ISTH-BAT data for women with Type 1 VWD, Type 3 VWD OC and age-matched female controls were used for comparison.
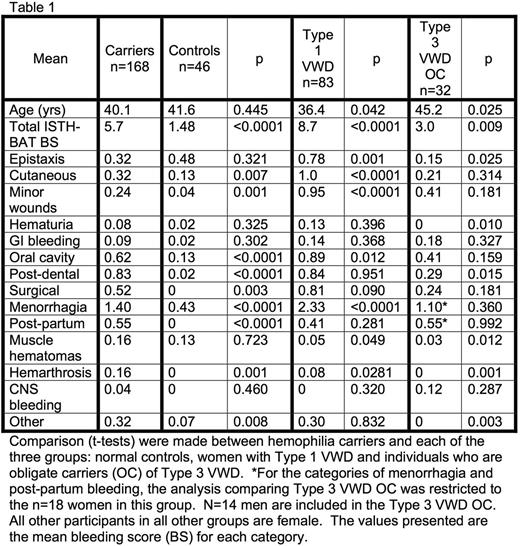
Results: A total of 329 participants were included in this study; 168 hemophilia carriers, 83 women with Type 1 VWD, 32 Type 3 VWD OC and 46 female normal controls. Hemophilia carriers and normal controls were similar in age (40.1 vs 41.6, p=0.445). The mean overall ISTH-BAT bleeding score (BS) was significantly higher in carriers than in controls (5.7 vs 2.48, p<0.0001). Carriers reported significantly more bleeding in the categories of cutaneous, minor wounds, oral cavity bleeding, post-dental bleeding, surgical bleeding, menorrhagia, post-partum bleeding and other when compared with controls. Carriers were older than Type 1 VWD patients (40.1 vs 36.4 years, p=0.042). While women with Type 1 VWD had higher total ISTH-BAT BS (8.7 vs 5.7, p<0.0001) as well as higher scores for epistaxis, cutaneous bleeding, minor wounds, oral cavity bleeding and menorrhagia, hemophilia carriers had significantly higher scores for muscle hematomas and hemarthrosis. Carriers were younger than Type 3 VWD OC (40.1 vs 45.2 years, p = 0.02), had higher overall ISTH-BAT BS (5.7 vs 3.0, p=0.009) and reported more bleeding in the following categories: total score, epistaxis, hematuria, dental, muscle hematomas, hemarthrosis, and other. In fact, hemophilia carriers reported more musculoskeletal bleeding than all other groups. Importantly, given the concern about over-reporting of joint bleeds by hemophilia carriers because of familiarity with hemarthrosis in affected male relatives, no Type 3 VWD OC reported joint bleeds. See Table 1 for detailed results.
Conclusion: In summary, our study showed that hemophilia carriers report significantly more bleeding by overall ISTH-BAT BS than age-matched female controls. Carriers experience both mucocutaneous bleeding as well as musculoskeletal bleeding. They score higher for mucocutaneous bleeding when compared with controls and when compared with Type 3 VWD OC. Overall Type 1 VWD patients experience more severe mucocutaneous bleeding than hemophilia carriers. However, hemophilia carriers report more musculoskeletal bleeding in the form of hemarthrosis and hematomas than all other groups. A comparison of overall ISTH-BAT BS between groups shows that bleeding in women with Type 1 VWD > hemophilia carriers > Type 3 VWD OC > controls. Additional research into the underlying pathophysiology of this abnormal bleeding is a critical next step in understanding and determining how to appropriately manage these patients.
Bidlingmaier:Novo Nordisk: Honoraria; Sobi: Honoraria; Pfizer: Honoraria; Biotest: Honoraria; Baxalta: Honoraria; Bayer: Honoraria; CSL Behring: Honoraria, Research Funding. Mingot-Castellano:Amgen: Consultancy; Pfizer: Consultancy; Novo Nordisk: Consultancy, Research Funding; Baxalta: Consultancy, Research Funding; Novartis: Consultancy; Bayer: Consultancy, Research Funding. Chitlur:Novo Nordisk: Consultancy; Baxalta: Honoraria; Bayer: Honoraria; Biogen-Idec: Honoraria; Pfizer: Honoraria. Fogarty:Bayer Healthcare: Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxter/Baxalta: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biogen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Pfizer: Employment, Membership on an entity's Board of Directors or advisory committees, Research Funding; Spark Therapeutics: Research Funding. Cuker:T2 Biosystems: Research Funding; Genzyme: Consultancy; Biogen-Idec: Consultancy, Research Funding; Amgen: Consultancy; Stago: Consultancy. Mancuso:Bayer Healthcare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Baxalta: Consultancy, Speakers Bureau; CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau; Sobi/Biogen Idec: Consultancy, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kedrion: Consultancy. Holme:Baxalta, now part of Shire: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Investigator Clinical Studies. Mathew:Bayer: Employment. James:CSL Behring: Research Funding; Octapharma: Research Funding; Biogen: Consultancy; Basalt: Consultancy; Bayer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal