Abstract
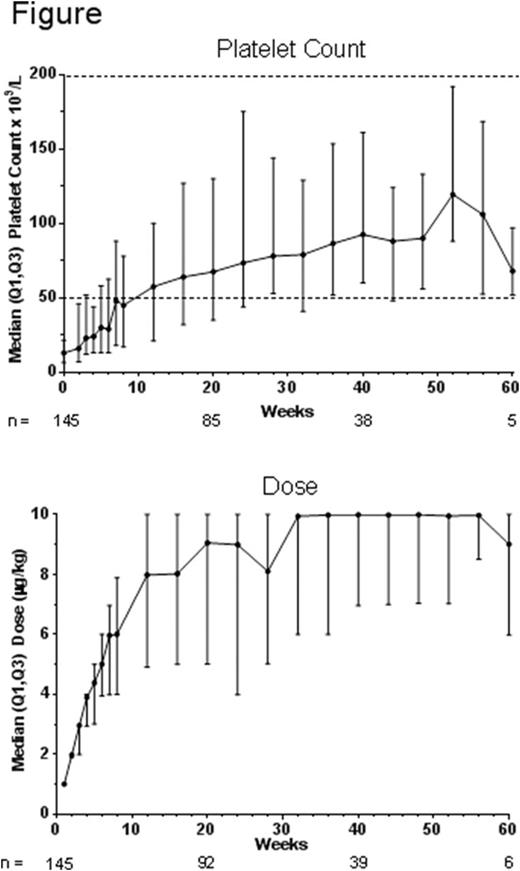
Background: The TPO receptor agonist romiplostim is approved for use in adults with chronic ITP. The use of romiplostim in children with ITP has been evaluated in phase 1/2 and 3 studies. In this study, children with ITP will receive open-label SC romiplostim for up to 3 years (y). Methods: Eligible children, recruited in 16 countries worldwide, had ITP for ≥6 months, ≥1 prior ITP therapy, and platelet (plt) count ≤30×109/L. Weekly SC dosing started at 1 μg/kg and was titrated in 1 μg/kg increments up to 10 μg/kg to target plt counts of 50-200×109/L. In patients in Europe, bone marrow aspirates and biopsies were obtained at baseline and after 1 or 2 y of exposure. The 1º endpoint was the % of time in the first 6 months with a plt response, with response defined as a plt count ≥50×109/L without rescue medication use in the past 4 weeks (wk). Results: As of 15 Mar 2016, 147 patients enrolled; 145 received ≥1 romiplostim dose. At baseline, median (min-max) age was 10 (2-17) y; 51% were female; 4% had prior splenectomy. Median (min-max) ITP duration was 1.9 (0.5-12.3) y and plt count was 13 (2-168)×109/L. The median (Q1, Q3) % of time with a plt response in the first 6 months patients were on study was 50% (0%, 83.3%); that of months 7-12 was 92% (33%, 100%). Over the course of the study, 80% (114/143) of patients had a plt response. The median (Q1, Q3) % of time with an increase in plt counts ≥20×109/L above baseline was 60% (25%, 84%). The median dose increased to 10 μg/kg by wk 32 (Figure). Median (min-max) treatment duration as of data cutoff was 25 (1-67) wk for a total exposure to date of 79 patient-years; 67 (46%) patients (or caregivers) self-administered romiplostim. Median (min-max) average weekly romiplostim dose was 6.1 (0.4-9.0) µg/kg. Thirty-two patients (22%) discontinued treatment for lack of efficacy (n = 17), required other therapy (n = 5), patient request (n = 4), noncompliance (n = 2), adverse event (AE) (n = 2) (interstitial lung disease unrelated to treatment in a 15 y old boy and abdominal pain, vomiting, and headache related to treatment per investigator in a 9 y old girl), administrative decision (n = 1), and investigator decision (n = 1). After wk 12, median plt counts remained ≥50×109/L (Figure). Thirty-four (23%) patients received rescue medications. The most frequently reported AEs were headache (27.6%), epistaxis (22.8%), and nasopharyngitis (23%); 15 (10.3%) patients had serious AEs (SAEs) including epistaxis (n = 4), petechiae (n = 2), decreased plt count (n = 2), and thrombocytopenia (n = 2). A case of abdominal pain was the only SAE deemed treatment-related by the investigator. Bleeding over the course of the study was seen in 52% of patients. CTCAE grade 3 bleeding was seen in 8 patients (6%) and included epistaxis (n = 5), ecchymosis (n = 2), petechiae (n = 2), and 1 case each of hematemesis, hematoma, SC hemorrhage, injection site hemorrhage, and mouth hemorrhage. No grade 4 or 5 bleeding was observed. One event of grade 2 phlebitis/thrombophlebitis in deep veins in the left arm lasting 14 days was reported in a 13 y old girl; plt counts were 40-70×109/L and the dose was 7-8 µg/kg during this time. Per investigator, this event was not serious or related to romiplostim. She was treated with antibiotics (oral amoxicillin/clavulanic acid and transdermal muciprocin) and continued romiplostim treatment as before. No neutralizing antibodies against romiplostim or TPO were identified. Of 30 patients with baseline bone marrow biopsies [50% female, median (min-max) age 10.5 (6-12) y, ITP duration 3.1 (0.6-11) y], all had modified Bauermeister scores of grade 0 (no reticulin) or 1 (fine fibers) and bone marrow typical for ITP. Of these 30 patients, 21 had evaluable on-study biopsies, with no increases in 2 or more grades, findings of collagen, or bone marrow abnormalities. Conclusion: In this year 1 datacut of an ongoing open-label study of romiplostim in children with ITP, the % of time in the first 6 months with a platelet response was 50%, with 80% of children having a platelet response at some point on study. The median romiplostim dose reached 10 μg/kg and there were no new safety signals. No effects of romiplostim were observed in the subset of patients with bone marrow biopsies. Future datacuts for year 2 and 3 in this study, the largest of romiplostim in children with ITP with 79 patient-years of exposure to date, will provide more information on platelet response, dose requirements, and safety.
Grainger:Baxter: Honoraria, Research Funding; Amgen Inc.: Honoraria; Novartis: Honoraria; GlaxoSmithKline: Honoraria. Bussel:Shionogi: Membership on an entity's Board of Directors or advisory committees; Symphogen: Membership on an entity's Board of Directors or advisory committees; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Research Funding; BiologicTx: Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prophylix Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protalex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Physicians Education Resource: Speakers Bureau; Genzyme: Research Funding; UpToDate: Patents & Royalties; Momenta Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Immunomedics: Research Funding; Cangene: Research Funding; Sysmex: Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cooper:UK ITP support association: Research Funding; National Organization for Rare Disorders: Research Funding; Imperial College BRC: Research Funding; Eisai: Consultancy, Honoraria, Other: Received honoraria for speaking at educational meetings; GlaxoSmithKline: Consultancy, Honoraria, Other: Received honoraria for speaking at educational meetings ; Amgen Inc.: Consultancy, Honoraria, Other: Received honoraria for speaking at educational meetings . Tarantino:Pfizer: Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Grifols: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Biogen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Baxalta: Membership on an entity's Board of Directors or advisory committees. Blanchette:Baxter: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data safety monitoring boards , Research Funding; Bayer Healthcare: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Octapharma: Other: Data safety monitoring boards . Carpenter:Amgen Inc.: Employment, Equity Ownership. Eisen:Amgen Inc.: Employment, Equity Ownership. Mehta:Amgen Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal