Abstract
Background: Patients (pts) with hematological disorders such as transfusion-dependent thalassemia (TDT) and myelodysplastic syndromes (MDS) require long-term supportive therapy with iron chelation therapy (ICT) to remove excess iron and prevent organ failure. Adherence to ICT can affect survival, so a well-tolerated and effective chelation regimen is required. The once-daily dispersible tablet (DT) formulation of the iron chelator deferasirox (DFX) has been available since 2005, offering an alternative to parenteral deferoxamine, yet barriers remain to pt adherence including the need to take the drug in a fasting state, palatability and GI tolerability. A DFX film-coated tablet (FCT) was developed that can be taken orally, once-daily with or after a light meal, and is potentially less burdensome to pts than the DT formulation. Here we report pt-reported outcomes, during the 24-week (wk) study.
Methods:ICT-naïve or pre-treatedpts aged ≥10 yrs, requiring ICT at DFX DT ≥30 mg/kg/day (TDT) or ≥20 mg/kg/day (MDS), with serum ferritin (SF) >1000 ng/mL, were enrolled. ICT-naïve pts received either DFX DT 20 mg/kg/day or DFX FCT 14 mg/kg/day. ICT pre-treated pts received a DT or FCT dose equivalent to their pre-washout dose. Dose was adjusted based on SF and investigator's judgment after wk 4 for ICT-naïve pts and after 3 months for ICT pre-treated pts (±5-10 mg/kg/day, maximum 40 mg/kg/day [DT]; ±3.5-7 mg/kg/day, maximum 28 mg/kg/day [FCT]); dose adjustments for safety reasons permitted at any time. The modified satisfaction with iron chelation therapy (modified SICT; 5-point response scales to assess adherence (six questions), satisfaction/preference (two questions), and concern (three questions) domain scores) and palatability (taste, aftertaste, ability to consume medicine, perception of medicine) questionnaires were completed at wks 2, 3, 13, and end of treatment. The GI symptom diary consisted of five items (pain in your belly, nausea, vomiting, constipation, diarrhea) rated on an 11-point scale and was completed daily.
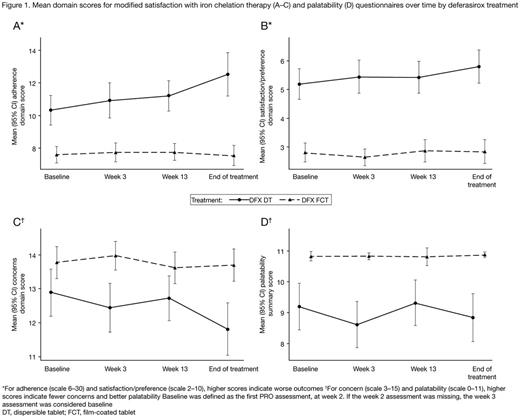
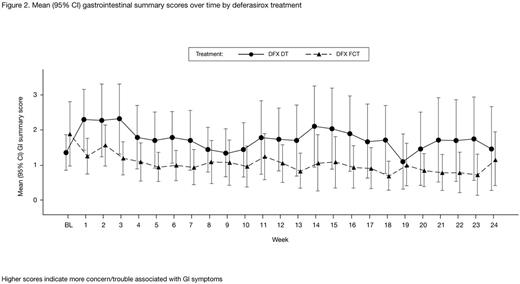
Results: In total, 173 pts were enrolled; 87 were treated with FCT and 86 were treated with DT. Completion rates for questionnaires (~80% at the start reducing to ~70% by wk 24) and GI daily diary (~70% at the start reducing to ~35% by wk 24) were similar for each formulation. Throughout the 24-wk study period, FCT pts consistently reported greater adherence (attributable to: easier to remember to take medication, thinking less about stopping medication, instructions from doctor followed more closely, medication easier to take, less bothered by time taken to prepare medication and waiting time before eating), greater satisfaction/preference (in general and with administration of medicine), and fewer concerns (attributable to: less worry about not swallowing enough medication, fewer limitations in daily activities, less concern about side effects), than DT pts (Figure 1). Pts assessed which medication for iron overload they would like best, choosing between: FCT, DT, powder to sprinkle on food, and 'I don't know'. At end of treatment, 53/60 (88.3%) evaluable pts receiving FCT indicated that they preferred the FCT and 41/63 (65.1%) evaluable pts receiving DT indicated they would prefer FCT. FCT pts reported higher satisfaction on palatability scores than DT pts (Figure 1), reporting no taste or aftertaste and that they are able to swallow the full amount of medicine with the right amount of liquid. The difference in score between DT and FCT was >1 point (minimal important difference) for all domains and palatability at most visits, indicating a clinically meaningful difference between formulations. Overall GI summary scores were low for both formulations indicating pts experience very little trouble/concern associated with GI symptoms; DT pts reported more trouble/concern than those receiving FCT (Figure 2).
Conclusions: These results show a clear preference in favor of DFX FCT in all domains for the modified SICT. Pts were satisfied with their medicine during the study period and more pts were satisfied with DFX FCT compared with DT at all visits. DFX FCT offers pts an improved formulation that does not require administration in a fasting state, has better palatability, and very little concern associated with GI tolerability. Enhanced pt satisfaction with the new DFX FCT formulation may improve adherence, thereby, reducing iron overload-related complications.
Taher:Novartis: Honoraria, Research Funding; Celgene: Research Funding. Origa:Novartis: Honoraria; Apopharma: Honoraria. Kouraklis:Novartis: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Gilead: Consultancy; Roche: Consultancy; Celegene: Consultancy. Kattamis:Novartis: Honoraria, Research Funding; ApoPharma: Honoraria. Cortoos:Novartis: Employment. Huang:Novartis: Employment. Weill:Novartis: Employment. Herranz:Novartis: Employment. Porter:Bluebird Bio: Consultancy; Agios Pharmaceuticals: Consultancy, Honoraria; Celegene: Consultancy; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal