Abstract
Background. Oral cavity involvement by chronic graft-versus-host disease (cGVHD) has a major impact on morbidity and health-related quality of life. Topical therapy for oral chronic GVHD (oGVHD) is an important part of the treatment armamentarium to control symptoms and reduce systemic immunosuppression. However, evidence-based treatments are scarce. Clobetasol is a class I superpotent synthetic glucocorticoid that has been used off-label in ointment form to treat refractory oGVHD. Application challenges and adherence to oral mucosa limit efficacy. Here, through a phase 2 clinical trial (NCT01557517), we tested the safety and efficacy of oGVHD therapy using clobetasol 0.05% solution formulated under an FDA-monitored IND as an oral rinse in aqueous base to facilitate dispersion and adherence in the oral cavity.
Methods. This was a phase 2 open label trial with an initial 2-week randomized double blind placebo-controlled period. The primary endpoint is the day 28 oGVHD response on the 273-point Oral Mucositis Rating Scale (OMRS). Consenting subjects were randomized to receive clobetasol or placebo rinse for 2 weeks, after which all patients (pts) received active rinse until completion of 28 days of treatment. Eligibility required an OMRS score of ≥20 with moderate erythema or ulceration, and stable/tapering systemic therapy during preceding two weeks and for duration of the blinded period. The dose schedule included a 2-minute swish with 10 ml of clobetasol rinse 3 times daily, and a once daily swish with nystatin (100,000 u/ml) rinse for anti-fungal prophylaxis. Systemic pneumocystis, antiviral, and antifungal prophylaxis were maintained during the study per NIH cGVHD guidelines. Patient-reported data, baseline and day 28 oral biopsies, salivary gland functional assessments, pharmacokinetic testing, baseline and day 28 ACTH stimulation tests, and immunologic outcomes, were also collected.
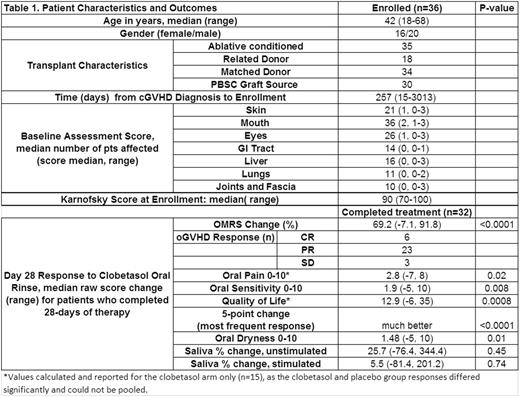
Results. Between 2012 and 2016, 32 of 36 randomized pts (16/36 female), median age 42y (18-68) completed 28 days of clobetasol rinse use. Pts were a median of 257 (15- 3013) days from cGVHD diagnosis, and had a median 4 organs involved. Six pts had no prior oral topical therapy. Pts with a treatment history had used a median of 2 prior oral topical agents, and 11 had previously used oral clobetasol ointment.
Clobetasol rinse reduced oGVHD: median baseline OMRS score 48 (24-85) vs. 14 (5-60) on day 28 (69% reduction, p<0.0001); 19% pts met criteria for complete response (CR) of oral cGVHD, 72% for partial response (PR), and 9% had stable disease (SD). Severity of histopathologic diagnosis was significantly reduced (p=0.0001) using the 2005 NIH cGVHD consensus histopathology grading criteria. Of pts with prior oral clobetasol use, 2/11 had CR, 8 had PR, and 1 had SD. Pts had significant improvements after 28 days in patient-reported oral pain* (p<0.05), oral sensitivity (p<0.01), OHIP-14 oral health related quality of life* (p<0.001), on a 5-point interval change scale (p<0.0001) and subjective oral dryness (p=0.01). However, no change was observed in stimulated or unstimulated saliva production. During the 14-day blinded period, the placebo group had a smaller mean improvement in OMRS score, 7%, than did the clobetasol group, 41%, p=0.003.
There were 4 withdrawals before day 28 (1 non-tolerance of study agent, 2 logistical issues, 1 death unrelated to study). Adverse events possibly/probably related to clobetasol included 3 PCR-confirmed cases of HSV reactivation (grade, gr 2-3), 3 oral candidiasis (gr 2), 1 other oral viral infection (gr 2), 6 gr 1 and 1 gr 2 cases of adrenal suppression, and three gr 1 facial edema.
Only small amounts (0-1.2ng/ml) of clobetasol were detectable in the bloodstream at day 28, however reduction in response to ACTH stimulation tests was noted. Frequency of peripheral CD4+, CD8+, B cells, NK cells, Th17 and Treg cells did not change during the treatment period. There were also no significant changes in production of interferon γ by circulating CD4+ or CD8+ T cells following PMA and ionomycin stimulation, suggesting no significant systemic immunosuppressive effects.
Conclusion. These controlled study data suggest that 0.05% clobetasol oral rinse is effective and safe for the treatment of oGVHD even in patients who failed prior clobetasol ointment. These data support the further development of 0.05% clobetasol oral rinse for oGVHD therapy.
Funding. NIH Intramural Research Program.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal