Abstract

Background: Tyrosine kinase inhibitors have become the treatment of choice for CML. However, the high cost and need for life-long treatment contribute to non-adherence and represent a major challenge in their use. Allogeneic HCT is potentially curative, but the very long-term health of the survivors is not known. It is also not clear whether a subgroup of CML patients carries a relatively low risk of long-term morbidity.
Methods: We addressed these gaps by studying long-term outcomes in 637 CML patients treated with allogeneic HCT between 1981 and 2010 at City of Hope or Univ MN, and surviving for at least 2y after HCT (median follow-up: 16.7y from HCT); 80% of the cohort was <45y at HCT; 68% received HCT in 1st chronic phase (CP); 63% received matched related [MRD], 34% matched unrelated donor (MUD) and 3% non-myeloablative HCTs; 79% received TBI; 65.8% developed chronic GvHD. Vital status information was collected as of May, 2016, using medical records, National Death Index and Lexis Nexis. US mortality rates were obtained from CDC's National Center for Health Statistics. Thirty percent (n=192) died after having survived at least 2 years after HCT; median time between HCT and death was 8.3y. Of the 445 patients alive at study, 288 (65%) completed the BMTSS health questionnaire used to examine the risk of CTCAE grade 3 (severe) or 4 (life-threatening) chronic health conditions. A sibling comparison group (n=404) also completed the BMTSS questionnaire.
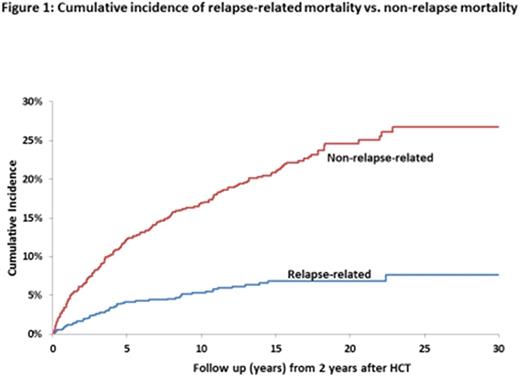
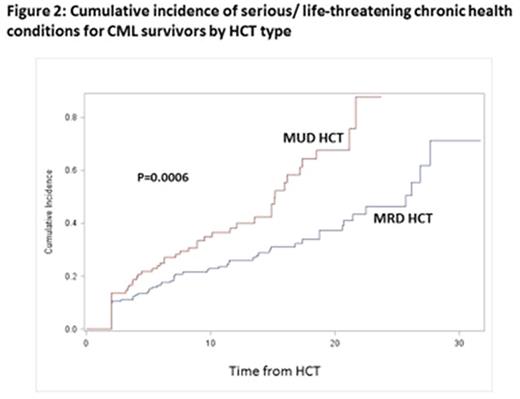
Results:Late Mortality: Overall survival was 72.1% at 20y and 69.9% at 30y from HCT. The 20y cumulative incidence of relapse-related mortality was 3.9% (95% CI, 2.6-5.8%) and of non-relapse-related mortality was 18.2% (95% CI, 19.8-28.1%) (Figure 1). 20y cumulative incidence of mortality by cause of death was as follows: infection (7%), chronic GvHD (6%), subsequent malignant neoplasms (SMNs: 3%). HCT recipients were at 4.4-fold increased risk of death (95% CI, 3.8-5.1, p<0.0001) than age-, race-, and sex-adjusted normal populations. For patients transplanted in 1st CP and surviving 15y, mortality rates became comparable with the general population (SMR, 1.5, 95% CI, 0.9-2.3, p=0.1). Among CML patients receiving HCT at <45y with Bu/Cy (n=70), overall survival was 81.5% at 20y from HCT; the 20y cumulative incidence of relapse-related mortality was 2.9% and of non-relapse-related mortality was 14%. This cohort was at 3.3-fold higher risk of death when compared with the general population (95% CI=1.7-5.7, p<0.0001). Late Morbidity: The 20y cumulative incidence of a severe/life-threatening chronic health condition among HCT survivors was 47.2% (95% CI, 39.0-54.9%); the incidence was higher (p=0.0006) for MUD vs. MRD recipients (Figure 2). After adjusting for age, sex, race and SES, HCT survivors were at 2.7-fold higher risk for severe/life-threatening chronic health conditions as compared with siblings (95% CI, 1.8-3.9, p<0.0001). The 20y cumulative incidence of specific conditions experienced by survivors and siblings were: SMNs (10.1% vs. 1.7%, p<0.001); diabetes (11.1% vs. 1.5%, p<0.001) and coronary artery disease (6.9% vs. 3.2%, p<0.001). CML patients receiving MRD HCT at <45y with Bu/Cy were not at increased risk of severe/life-threatening chronic health conditions when compared with the sibling comparison group (HR=0.81, 95% CI, 0.26-2.54, p=0.7).
Conclusions: Conditional on surviving the first 2y after HCT, the overall survival exceeds 70% at 20y and remains stable at 70% at 30y after HCT. Non-relapse related mortality (infections, chronic GvHD, SMNs) is by far the major contributor to the late mortality. Conditional on surviving the first 15y, mortality rates are similar to those observed in the general population. HCT survivors are at a 2.7-fold higher risk of severe/life-threatening morbidity when compared with siblings. The more common morbidities include SMNs, diabetes and coronary artery disease. However, CML patients receiving HCT at <45y with Bu/Cy conditioning enjoy survival rates exceeding 81% at 20y from HCT, and their burden of long-term morbidity is comparable to that experienced by siblings. These findings could help inform decisions regarding therapeutic options for management of CML.
Snyder:BMS: Membership on an entity's Board of Directors or advisory committees; Ariad: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Forman:Mustang Therpapeutics: Other: Construct licensed by City of Hope.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal