Abstract
Introduction
The tyrosine kinase inhibitor (TKI), imatinib, dramatically improves the prognosis of chronic myelogenous leukemia (CML) by suppressing the function of BCR-ABL gene. It was shown that imatinib could be discontinued in a proportion of patients with CML who maintained deep molecular response (DMR) for at least 2 years. (Lancet Oncol. 2010; 11:p1029) Treatment with second generation TKI, dasatinib, after imatinib resistance/intolerance could also be discontinued after maintaining DMR for over 1 year in a proportion of patients with CML (Lancet Haematol. 2015; 2:p528). In this Japanese prospective multicenter trial (D-STOP trial by Shimousa Hematology and Kanto CML Study Groups, ClinicalTrials.gov Identifier: NCT01627132), we aimed to discontinue dasatinib in patients with CML who maintained DMR for over 2 years.
Methods:
Chronic phase CML patients treated with TKIs who had undetectable BCR-ABL1 mRNA were enrolled. After confirmation of undetectable BCR-ABL1 mRNA (International Scale <0.01%) using real-time quantitative polymerase chain reaction (RQ-PCR) in the central laboratory, the patient received additional dasatinib treatment for another 2 years as consolidation therapy. Patients who maintained DMR during the consolidation therapy proceeded to discontinue dasatinib. BCR-ABL1 mRNA was monitored every month in year 1 and every 3 months in year 2. Molecular relapse was defined as two successive positive RQ-PCRs for BCR-ABL1 within 1 month. The relapsed patients restarted dasatinib. The primary endpoint was treatment-free survival after 12 months of discontinuation. Lymphocyte subsets were analyzed using flow cytometry during and after the consolidation therapy.
Results:
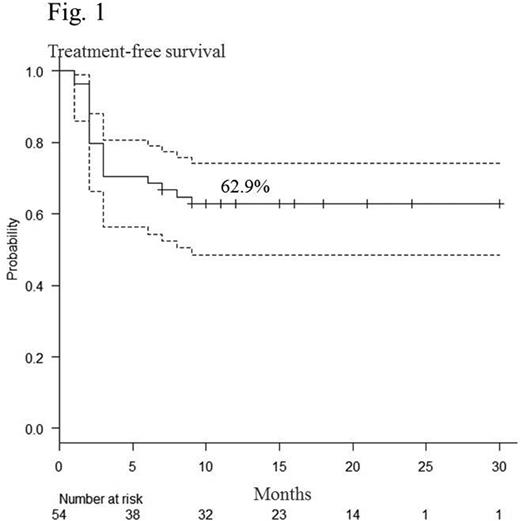
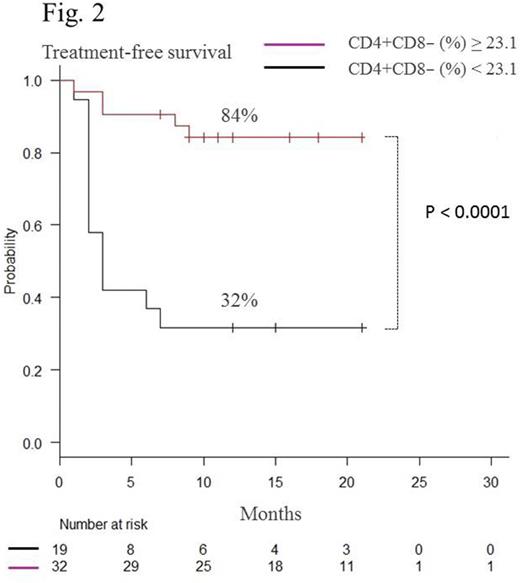
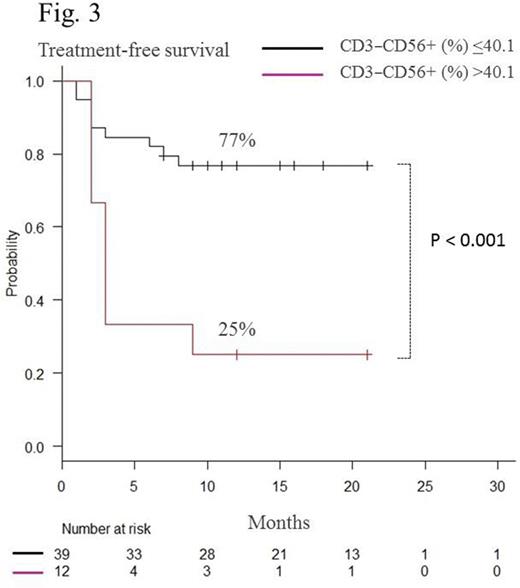
Sixty-five patients received consolidation therapy, and 54 discontinued dasatinib treatment after maintenance of DMR for 2 years. Mean age of the patients was 54.2 (25-82) years, and median follow-up period after cessation of dasatinib was 16.2 (7-30) months. Twenty patients relapsed during the observation period. Using Kaplan-Meier analysis, the estimated overall treatment-free survival (TFS) was 62.9% (48.5-74.2) at 12 months (Fig.1). Most relapses occurred within 6 months after discontinuation of dasatinib. All relapsed patients responded again to dasatinib. There was no significant difference either in estimated TFS between males and females or Sokal scores at diagnosis. During the consolidation therapy, the proportion (%) of CD4+CD8−, CD3−CD56+, CD16+CD56+, and CD57+CD56+ cells among total lymphocytes were monitored using flow cytometry in patients who could discontinue dasatinib during the observation periods (group A) and those who relapsed (group B). At the start of the consolidation therapy, there was no significant difference between groups A and B (CD4+CD8−%: 33.6 vs 34.0, p = 0.89, CD3−CD56+%: 24.3 vs 24.1, p = 0.95; CD16+CD56+%: 22.1 vs 23.9, p = 0.57; CD57+CD56+%: 19.7 vs 21.8, p = 0.51, respectively). In group B, the proportion of CD4+CD8− cells gradually decreased, whereas CD3−CD56+, CD16+CD56+, and CD57+CD56+ cells gradually increased during the consolidation therapy. All four types of cells were relatively stable in group A. At the end of the consolidation therapy, there was a significant group difference in the proportion of these subsets (CD4+CD8−%: 29.6 vs 22.2, p = 0.018*; CD3−CD56+%: 25.9 vs 37.3, p < 0.01*; CD16+CD56+%: 23.2 vs 34.4, p < 0.01*; CD57+CD56+%: 21.9 vs 32.1, p < 0.001*; respectively). We concluded that patients with CD4+CD8− cells ≥23.1%, CD3−CD56+ cells ≤40.1%, CD16+CD56+ ≤35.6% or CD57+CD56+ ≤26.6% at the end of the consolidation therapy had significantly higher estimated overall TFS at 12 months than those without each condition (CD4+CD8−%: 84% vs 32%, p < 0.0001* (Fig.2); CD3−CD56+%: 77% vs 25%, p < 0.001* (Fig.3), CD16+CD56+%: 77% vs 25%, p < 0.0001*; CD57+CD56+%; 84% vs 46%, p < 0.01*, respectively). Although increased large granular lymphocytes and NK cells were reported to be associated with high responses to dasatinib (Int J Hematol. 2014; 99:p41), the unique profiles of lymphocyte subsets could predict successful discontinuation of dasatinib.
Conclusion:
Discontinuation of TKI in patients with chronic phase CML after consolidation therapy with dasatinib for 2 years was feasible with relatively high TFS. The unique profile of lymphocyte subsets might be able to predict successful discontinuation.
Kumagai:Bristol-Myers Squibb: Speakers Bureau; Novartis: Speakers Bureau; Pfizer: Speakers Bureau. Nakaseko:Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria; Pfizer: Honoraria, Research Funding. Nishiwaki:Novartis: Research Funding. Yoshida:Bristol-Myers Squibb: Honoraria, Speakers Bureau; Otsuka Pharmaceutical: Honoraria, Research Funding, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau. Morita:Bristol-Myers Squibb: Speakers Bureau. Sakamoto:Takeda Pharmaceutical: Consultancy; Yakult: Other: Remuneration. Inokuchi:Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria; Celgene: Honoraria; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal