Abstract
Current AML therapies are effective in a subset of patients, but often lead to prolonged myelosuppression. CD123 is an attractive AML target due to its elevated expression on AML compared to normal bone marrow cells. Still, severe myelosuppression and myeloablation have been reported in preclinical studies for some CD123-targeted therapies. Here, we present a novel ADC which selectively kills CD123-positive AML cells over normal bone marrow cells.
A novel humanized anti-CD123 antibody with two engineered cysteines for payload conjugation was generated. Indolinobenzodiazepine dimers, termed IGNs, were chosen as payload molecules for the antibody due to their high potency against AML cells. The IGN dimers containing mono-imines alkylate DNA, whereas the di-imine containing IGNs can both alkylate and crosslink DNA. To select an optimal IGN payload, we compared the cytotoxicity of an ADC with a mono-imine IGN (A-ADC) to one with a di-imine IGN (C-ADC) on AML cells, as well as normal bone marrow cells in vitro.
Potency of the ADCs was evaluated using AML cell lines that have CD123 levels similar to patient cells and carry markers of poor prognosis (FLT3-ITD , MDR1, EVI1, DNMT3A and TP53), as well as on samples from 11 AML patients. AML cells exposed to either ADC displayed markers of DNA damage, cell cycle arrest and apoptotic cell death by flow cytometry. Both ADCs were highly cytotoxic, generating IC50 values between 0.4 to 60 pM on the cell lines in WST-8 assays and killing 90 percent of progenitors from AML patients between 2 to 46 pM in CFU assays. The C-ADC was, on average, two-fold more active than the A-ADC. The cytotoxicity of both ADCs was CD123 dependent, since masking CD123 with a competing anti-CD123 antibody reduced the potency by more than 100-fold.
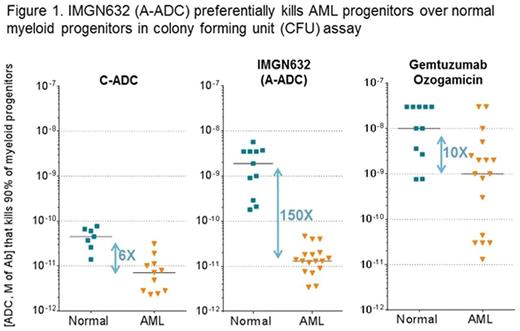
Toxicity of the ADCs was assessed using bone marrow cells from a healthy human donor. The cells were exposed to the ADCs at 100 pM (a concentration highly potent against all AML samples) for 72 hours, and then markers of apoptosis were detected in different cell populations by flow cytometry. Neither ADC affected the viability of monocytes, lymphocytes and multipotential progenitors, consistent with low CD123 levels in these cell populations. In contrast, an apoptotic signal was detected in myeloid progenitors, the population with the highest CD123 level, following exposure to the C-ADC, but not to the A-ADC. The toxicity of the ADCs was also tested in CFU assays on bone marrow cells from 7 healthy donors, as the assays have been reported to predict clinical myelosuppression. Surprisingly, the C-ADC was, on average, 50-fold more cytotoxic to normal myeloid progenitors than the A-ADC (40 pM vs 2,000 pM IC90 values, respectively) (Figure 1). Finally, we compared CD123 independent toxicity of the ADCs in CD-1 mice. The C-ADC showed significantly reduced tolerability, and unlike the A-ADC, was associated with delayed toxicity manifested by weight loss 30 days after administration. Based on its potent yet highly selective toxicity to AML cells and more favorable tolerability profile, the A-ADC was selected for further study, and renamed as IMGN632.
To compare IMGN632 to an ADC previously approved for the treatment of AML, the potency of IMGN632 and gemtuzumab ozogamicin (GO) was tested on bone marrow cells from 11 healthy donors and 17 AML patients, including 4 relapsed/refractory and 8 with strong multidrug resistance (Figure 1). Only 6 of 17 AML samples were sensitive to GO at concentrations that did not impact normal progenitors. In contrast, AML progenitors from all 17 patients were highly sensitive to IMGN632. Importantly, normal progenitors were only affected by IMGN632 at 150-fold higher concentrations. The pronounced difference between AML and normal progenitors in their sensitivity to IMGN632 likely reflects both higher CD123 levels on AML progenitors and the lower sensitivity of normal progenitors to the mono-imine IGN payload we observed in CFU assays.
In conclusion, through use of a mono-imine IGN payload, IMGN632 demonstrated potent activity in all tested AML samples at concentrations far below levels that impact normal bone marrow cells, suggesting the potential for efficacy in AML patients in the absence of or with limited myelosuppression. These findings together with strong efficacy in multiple AML xenograft models (Kovtun et al., 21st EHA congress, 2016; Adams et al., 58th ASH annual meeting, 2016) support advancing IMGN632 into clinical trials.
Kovtun:ImmunoGen, Inc.: Employment. Jones:ImmunoGen, Inc.: Employment. Audette:ImmunoGen, Inc.: Employment. Harvey:ImmunoGen, Inc.: Employment. Gerard:ImmunoGen, Inc.: Employment. Wilhelm:ImmunoGen, Inc.: Employment. Bai:ImmunoGen, Inc.: Employment. Adams:ImmunoGen, Inc.: Employment. Goldmacher:ImmunoGen, Inc.: Employment. Chari:ImmunoGen: Employment. Chittenden:ImmunoGen, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal