Abstract
Chemo-refractory acute myeloid leukemia (AML) is associated with poor prognosis and treatment options are extremely limited. Most of these patients are ineligible for allogeneic stem cell transplantation. Chemo-refractory AML is thought to arise due to selection pressure of resistant clones from prior use of chemotherapy or in some cases pre-exist due to properties of the leukemic stem cells (LSC). CLEC12A (also known as CLL1) has previously been described as being selectively over expressed in LSCs. Successful modalities to target CLEC12A and eradicate the LSC would overcome chemo-refractoriness in AML and would represent a vertical advance in the field.
In this study, we confirm that CLEC12A is heterogenously expressed on AML blasts and over-expressed on AML LSC. We also show that CLEC12A is overexpressed on bone marrows from patients with AML that fail to achieve a complete remission after induction chemotherapy, suggesting that it could be a marker for residual disease that is refractory to chemotherapy. We then separated AML blasts into CLEC12A positive or negative cells by magnetic sorting. CLEC12A positive blasts selected from AML patients were more resistant to chemotherapy compared to CLEC12A negative blasts (20% killing of CLEC12A positive AML cells versus 43% of CLEC12A negative AML cells when cultured with cytarabine 10 µg/ml, P=0.01). This finding was confirmed by using the AML MOLM14 cell line engineered to overexpress CLEC12A. CLEC12Ahigh MOLM14 cells were more resistant to chemotherapy compared to wild type MOLM14 cells (P=0.003). We then evaluated CLEC12A resistance to chemotherapy in a patient derived AML xenograft model. We found a relative increase in CLEC12A positive cells post Ara-C induction chemotherapy in AML xenograft models (Figure 1). The observation that CLEC12A positive cells are more resistant to chemotherapy provided a solid rationale to target CLEC12A with chimeric antigen receptor T (CART) cells. We therefore developed a second generation CLEC12A directed CAR construct using CD3z and 41BB costimulatory domains and generated CLEC12A CART cells by lentiviral transduction with this construct. Upon incubation with primary AML samples or AML cell lines, CLEC12A CART cells resulted in modest effector functions, due to the heterogeneity of CLEC12A expression on AML blasts. However when CLEC12A overexpressed MOLM14 cell line or CLEC12Apos selected leukemic cells were used as targets, CLEC12A-CART cells resulted in potent cytotoxicity, proliferation and cytokine production, indicating that CLEC12A-CART cells are more specific for LSC.
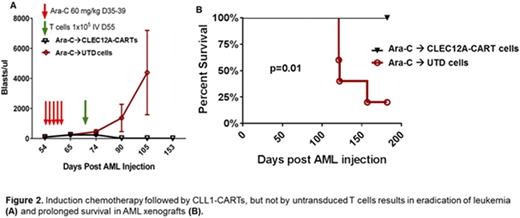
To test the in vivo anti-leukemic activity of CLEC12A CARTs, we used primary human AML blasts xenografted into NSG-S mice (NOD-SCID-γc-/-, additionally transgenic for human stem cell factor, IL3 and GM-CSF). Treatment with CLEC12A CART (single dose, 1x105 total T cells via tail vein injection) resulted in modest activity against AML when employed as monotherapy. To investigate the potential role of CLEC12A CART cells in eradication of MRD and LSC, mice were treated first with chemotherapy (cytarabine 60 mg/kg intraperitoneal injection daily for 5 days) followed by a single dose (1x105 total T cells via tail vein injection) of either CLEC12A CARTs or control untransduced T cells (UTD). Treatment with CLEC12A CART cells resulted in eradication of leukemia and prolonged survival in these mice (overall survival at 200 days of 100% after CLEC12A CARTs compared to 20% after UTD, p=0.01, Figure 2).
In conclusion, our preclinical studies reveal that CLEC12A positive cells in leukemia are resistant to chemotherapy and can be successfully targeted with CART cells. CLEC12A CART cells can potentially be employed as a consolidation regimen after induction chemotherapy to eradicate LSC and MRD in AML.
Kenderian:Novartis: Patents & Royalties, Research Funding. Ruella:novartis: Patents & Royalties: Novartis, Research Funding. Singh:Novartis: Employment. Richardson:Novartis: Employment, Patents & Royalties, Research Funding. June:Tmunity: Equity Ownership, Other: Founder, stockholder ; Immune Design: Consultancy, Equity Ownership; Novartis: Honoraria, Patents & Royalties: Immunology, Research Funding; University of Pennsylvania: Patents & Royalties; Celldex: Consultancy, Equity Ownership; Johnson & Johnson: Research Funding; Pfizer: Honoraria. Gill:Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal