Abstract
Background:
Classical Hodgkin lymphoma (HL) expresses surface CD30. Brentuximab vedotin (BV) is an antibody-drug conjugate that selectively delivers a potent cytotoxic agent, monomethyl auristatin E (MMAE), specifically to cells expressing surface CD30. Although BV elicits a high response rate (75%) in HL, patients who do not achieve complete response (CR) will eventually develop resistance to BV. We have developed 2 BV-resistant HL cell models and shown that resistance to BV is due to MDR1 upregulation rather than downregulation of surface CD30. MDR1 upregulation leads to increased drug exporter pump and decreased intracellular MMAE, the cytotoxic agent in BV. Our hypothesis is that by inhibiting MDR1, we can overcome resistance to BV and also achieve synergy with BV in HL cell lines and mouse xenograft model.
Methods:
HL cell lines (L428 and KMH2) were used for in vitro experiments. The selection of BV resistant cell model (L428R and KMH2) was done using pulsatile treatment with BV. Both BV resistant cell models were confirmed with MTS assay and cell growth assays. MDR1 upreguation was confirmed by qRT-PCR and western blot. Intracellular MMAE concentration was assessed by mass spectrometry. MDR1 inhibitors verapamil (VrP, 10 ug/ml) and cyclosporine (CsA, 5 uM) were chosen. NSG mice were used for mouse xenograft experiments.
Results:
MTS assay showed that the IC50 to BV increased 12 folds in L428R (32 ug/ml in L428, 391 ug/ml in L428R), and that the IC50 to BV has increased 17 folds in KMH2R (13 ug/ml in KMH2, 219 ug/ml in KMH2R). QRT-PCR shows that L428R and KMH2R had upregulation of MDR1 mRNA, and western blot shows L428R and KMH2R had overexpression of PgP (MDR1) as compared to L428 and KMH2. Mass spectrometry shows that the intracellular MMAE concentration was 7.6 folds lower in L428R as compared to L428 after treatment with BV. We then examined the effect of MDR1 inhibitors (VrP and CsA) on the IC50 of BV in L428R and KMH2R. The addition of VrP to L428R was able to decrease the IC50 from 344 ug/ml to 22.8 ug/ml (15 fold). The addition of VrP to KMH2R was able to decrease the IC50 from 170 ug/ml to 22 ug/ml (7.7 fold). The addition of CsA to L428R was able to decrease the IC50 from 275 ug/ml to 0.0068 ug/ml (40441 fold). The addition of CsA to KMH2 was able to decrease the IC50 from 129 ug/ml to 0.11 ug/ml (1098 fold). Also, the addition of VrP to L428R was able to increase the intracellular MMAE levels of L428R by 6.4 folds.
We then examined the effect of MDR1 inhibitors plus BV in BV naïve parental cell lines. The addition of VrP to L428 was able to decrease the IC50 of BV from 69 ug/ml to 0.03 ug/ml (2300 folds). The addition of VrP to KMH2 was able to decrease the IC50 of BV from 6.3 ug/ml to 0.0013 ug/ml (4846 folds). The addition of CsA to L428 was able to decrease the IC50 of BV from 36 ug/ml to 0.0085 ug.ml (4235 folds). The addition of CsA to KMH2 was able to decrease the IC50 of BV from 7.1 ug/ml to 0.0002 ug/ml (32645 folds). Neither the VrP or CsA had individual effects on HL parental or resistant cell lines.
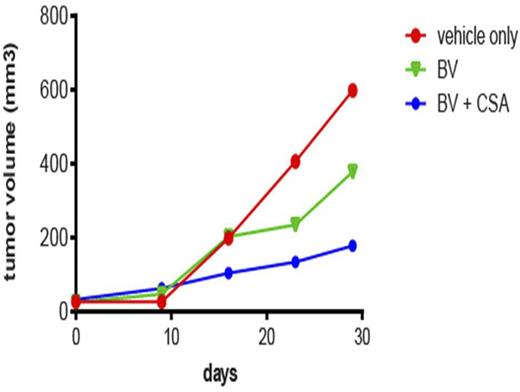
Because CsA seems to have the strongest effect in KMH2 cell lines, we examined the effect of CsA plus BV in KMH2 mouse xenograft model. KMH2 was injected into the right flank of the mice to form subcutaneous lymphomas and mice were separated into 3 groups of 5. Group 1 is control without addition of BV or CsA. Group 2 is control plus BV at dose of 0.3 mg/kg intravenously every 4 days. Group 3 is treated with BV at doses of 0.3 mg/kg intravenously every 4 days and CsA intraperitoneally at 15 mg BID. We show that group 2 had a 37% reduction in tumor volume as compared to group 1 and that group 3 had a 70% reduction in tumor volume as compared to group 1 (Figure 1).
Conclusion:
MDR1 upregulation increases efflux pump activity and decreases intracellular accumulation of MMAE, and leads to resistance to BV in HL. This mechanism of resistance can be overcome with MDR1 inhibitors VrP and CsA. The addition of MDR1 inhibitors to BV is able to increase the intracellular accumulation of MMAE, and restore the sensitive to BV. We are also able to show synergery between MDR1 inhibitors and BV in the parental non-resistant HL cell lines and mouse xenograft model. This is the first study showing synergy between MDR1 inhibition and antibody drug conjugates. A clinical trial has been initiated to examine the safety/efficacy of the combination of MDR1 inhibitors plus BV in patients with relapsed/refractory HL.
Chen:Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Merck: Consultancy, Research Funding; Millenium: Consultancy, Research Funding, Speakers Bureau; Genentech: Consultancy, Speakers Bureau. Kwak:Antigenics: Equity Ownership; Celltrion: Consultancy; XEME BioPharma: Consultancy, Equity Ownership; Sella Life Sciences: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal