Abstract
The transcription factor Runx1 is essential for definitive hematopoiesis, and human RUNX1 is frequently translocated or mutated in leukaemia. Runx1 expression from its two promoters, the proximal P1 and distal P2, must be tightly regulated for normal hematopoiesis to occur. The mechanism(s) of promoter-specific Runx1 expression during development and differentiation of hematopoietic progenitors is poorly understood. Gene regulatory elements located in non-coding DNA, such as the previously identified +24 enhancer (Ng et al, Stem Cells 2010), are likely to be important for Runx1 transcription. Here, we identify novel cis-regulatory DNA elements that are competent to drive hematopoietic expression in zebrafish, and that interact long-range with the Runx1 gene.
Circular chromosome conformation capture sequencing (4C-seq) was used to identify long-range interactions of DNA elements with the promoters of Runx1 in the mouse hematopoietic progenitor cell line, HPC7. We used the P1 and P2 promoters and the +24 enhancer (located 24 kb downstream from the P1 promoter) as anchor points from which to view cis-interactions on mouse chromosome 16. 4C-seq reveals that high levels of chromatin interactions at Runx1 are contained within a 1 megabase domain, spanning from the upstream Setd4 gene to the downstream Clic6 gene. A comparison with HiC data in K562 cells shows that the same domain is conserved in humans.
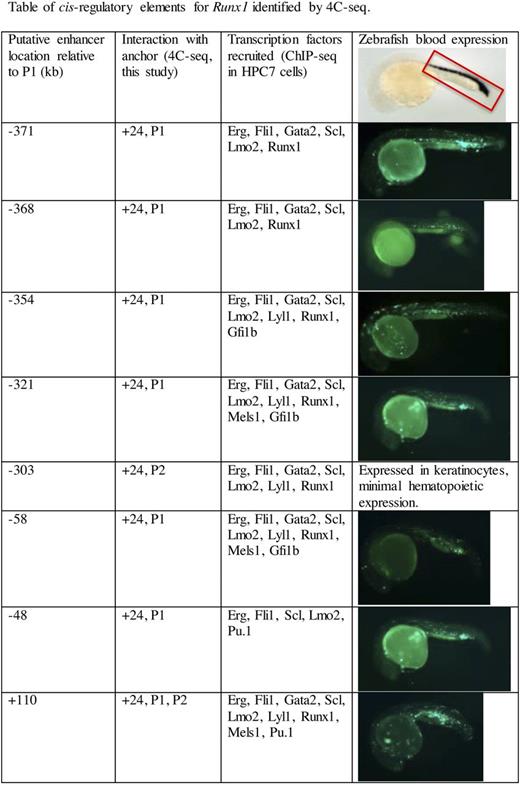
HPC7 cells express Runx1 almost exclusively from the P1 promoter, such that DNA elements interacting with P1 are likely to activate gene expression. The active P1 promoter interacts with multiple non-coding regions both upstream and downstream of Runx1-P1. Interacting regions were overlapped with DNAse hypersensitivity peaks and transcription factor binding sites (available in the USCS genome browser) to identify putative enhancers for Runx1. The majority of putative enhancer regions function as blood specific enhancers in zebrafish (Table). Interestingly, the previously identified +24 enhancer interacted promiscuously within and outside of the 1 megabase Runx1 domain, indicating it may regulate the expression of many additional genes. Overall, our study of the Runx1 interactome identifies multiple novel hematopoietic enhancers that directly interact with the P1 promoter of Runx1, suggesting they are likely involved in regulating Runx1 expression.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal