Abstract
Introduction: Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice, contributing directly to morbidity and mortality largely through thromboembolism events such as stroke. The risk of stroke in patients with AF is five times higher than in patients without AF. The pathophysiological of thromboembolism in AF is multifactorial and is not only related to stasis in a poorly contractile left atrium. Indeed, there is an increasing body of evidence to support that Virchow triad, including abnormal blood stasis, endothelial damage, and changes in blood constituents, is the main cause of thromboembolism or prothrombotic state in AF patients. However, relatively little is known about the precise role of phosphatidylserine (PS), one of the blood constituents, in the pro-thrombotic state or hypercoagulability of non-valvular AF (NVAF). Our objectives were to study the increased PS exposure on microparticles (MPs) and the outer membrane of MP-origin blood cells in NVAF patients, and to evaluate their procoagulant activity (PCA).
Methods: Our study included NVAF patients without (n = 60) and with left atrial thrombosis (n = 30) and healthy controls (n = 30). PS exposure on MPs and blood cells was analyzed with flow cytometry and confocal microscopy. PCA was evaluated using clotting time, extrinsic/intrinsic FXa and prothrombinase production, and fibrin formation assays. Inhibition assays of PCA of PS+ MPs and blood cells were performed using lactadherin.
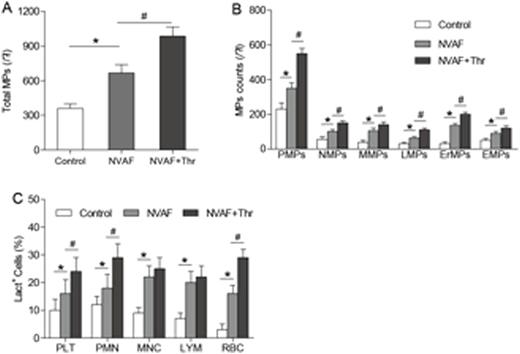
Results: The number of PS+ MPs was significantly higher in NVAF patients (1.8-fold, P < 0.001) and NVAF patients with left atrial thrombosis (2.7-fold, P < 0.001) compared with healthy subjects, respectively. Furthermore, the number of PS+ MPs in NVAF patients with left atrial thrombosis was significantly higher than that in NVAF patients without (1.4-fold, P < 0.01, Figure 1A). Moreover, the PS+ MPs were originated from platelets (CD41a+, PMPs), neutrophils (CD66b+, NMPs), mononuclear cells (CD14+, MMPs), lymphocytes (CD3/19+, LMPs), erythrocytes (CD235a+, ErMPs), and endothelial cells (CD31+ CD41a-, EMPs) (Figure 1B). The levels of PS+ platelets, neutrophils, mononuclear cells, lymphocytes, and erythrocytes were significantly higher (all P < 0.001) in each NVAF group than those in healthy controls. And the levels of PS+ platelets, neutrophils, and erythrocytes in NVAF patients with left atrial thrombosis were significantly higher than those in NVAF patients (all P < 0.01, Figure 1C). In addition, circulating PS+ MPs cooperated with PS+ blood cells, contributing to markedly shortened coagulation time and dramatically increased FXa/thrombin generation and fibrin formation in each NVAF group (all P < 0.01). Moreover, blockade of exposed PS on MPs and blood cells with lactadherin inhibited PCA by approximately 80%.
Conclusions: Our results suggest that increased PS exposure on MPs and blood cells play a procoagulant role in NVAF patients with or without left atrial thrombosis. Blockade of PS prior to left atrial thrombosis could become a novel therapeutic strategy to prevent thrombosis in these patients.
Flow cytometry analyses of PS+ MPs and blood cells in each NVAF group and control subjects. (A and B) Events were selected for lactadherin-Alexa Fluor 488 binding. Lactadherin-positive MPs were further examined for expression of other antigens by co-labeling with Alexa Fluor 488- and Alexa Fluor 647-labeled antibodies as follows: PMPs (Alexa Fluro 647-CD41a+), NMPs (Alexa Fluro 647-CD66b+), MMPs (Alexa Fluro 488-CD14+), LMPs (Alexa Fluor 647-CD3+/Alexa Fluor 488-CD19+), ErMPs (Alexa Fluor 488-CD235a+), and EMPs (Alexa Fluro 488-CD31+/Alexa Fluor 647-CD41a-). (C) Lactadherin-binding counts of PLT/PMN/MNC/LYM/RBC from controls (n = 30), NVAF patients without (n = 60) and with left atrial thrombosis (n = 30) were measured. MPs, microparticles; NVAF, non-valvular atrial fibrillation; Thr, thrombosis; PMPs, platelet-derived MPs; NMPs, neutrophil-derived MPs; MMPs, mononuclear cell-derived MPs; LMPs, lymphocyte-derived MPs; ErMPs, erythrocyte-derived MPs; EMPs, endothelial-derived MPs; PLT, platelet; PMN, polymorphomuclear cells; MNC, mononuclear cell; LYM, lymphocyte; RBC, red blood cell. *P <0.001 vs. controls. #P< 0.01 vs. NVAF with Thr.
Flow cytometry analyses of PS+ MPs and blood cells in each NVAF group and control subjects. (A and B) Events were selected for lactadherin-Alexa Fluor 488 binding. Lactadherin-positive MPs were further examined for expression of other antigens by co-labeling with Alexa Fluor 488- and Alexa Fluor 647-labeled antibodies as follows: PMPs (Alexa Fluro 647-CD41a+), NMPs (Alexa Fluro 647-CD66b+), MMPs (Alexa Fluro 488-CD14+), LMPs (Alexa Fluor 647-CD3+/Alexa Fluor 488-CD19+), ErMPs (Alexa Fluor 488-CD235a+), and EMPs (Alexa Fluro 488-CD31+/Alexa Fluor 647-CD41a-). (C) Lactadherin-binding counts of PLT/PMN/MNC/LYM/RBC from controls (n = 30), NVAF patients without (n = 60) and with left atrial thrombosis (n = 30) were measured. MPs, microparticles; NVAF, non-valvular atrial fibrillation; Thr, thrombosis; PMPs, platelet-derived MPs; NMPs, neutrophil-derived MPs; MMPs, mononuclear cell-derived MPs; LMPs, lymphocyte-derived MPs; ErMPs, erythrocyte-derived MPs; EMPs, endothelial-derived MPs; PLT, platelet; PMN, polymorphomuclear cells; MNC, mononuclear cell; LYM, lymphocyte; RBC, red blood cell. *P <0.001 vs. controls. #P< 0.01 vs. NVAF with Thr.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal