Abstract
Background: Allogeneic hematopoietic cell transplantation (HCT) is an important treatment for adults with acute lymphoblastic leukemia (ALL). The combination of cyclophosphamide with total body irradiation (TBI) at doses of 1200 to 1500 cGy (Cy/TBI1200) has been used in most prospective trials of HCT in ALL. However, TBI is associated with significant short and long term toxicities. Although Cy/TBI/1200 is the standard of care at most Canadian transplant centers one center instead prefers a conditioning regimen including fludarabine, busulfan, and low dose TBI (400 cGy) (Flu/Bu/TBI400) (Daly et al. BBMT 2012). We sought to compare the outcomes of HCT for adults with ALL who routinely received either Cy/TBI1200 or Flu/Bu/TBI400 as their conditioning regimen with the hypothesis that the regimens will result in similar outcomes. Conditioning regimen was highly correlated with transplant center, minimizing any selection bias in which clinicians might choose a regimen perceived to be of lower toxicity for selected patients.
Methods: We included patients aged 18 or above who underwent a first myeloablative HCT for ALL in complete remission at one of three Canadian transplant centers from 2004-2014. Two of the centers use Cy/TBI1200 as a preferred conditioning regimen and the third center preferred Flu/Bu/TBI400. As the choice of conditioning regimen is strongly linked to transplant center we have performed an instrumental variable analysis in which the instrumental variable was the transplant center preference. Other transplant practices, including indications for transplant and supportive care, were similar between the centers. Overall survival (OS), progression-free survival (PFS), relapse rate (RR), and non-relapse mortality (NRM) were compared between groups.
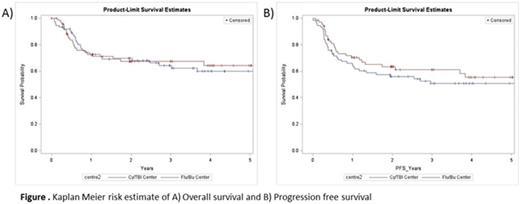
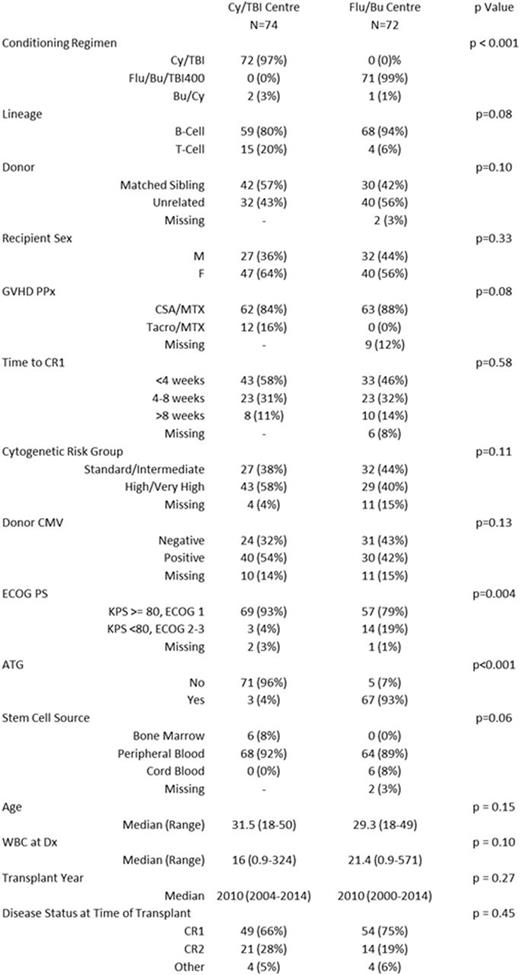
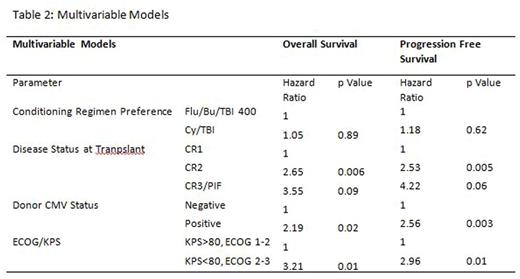
Results: A total of 146 patients were included, with 74 treated at centers that prefer Cy/TBI1200, of whom 72 received Cy/TBI1200 as conditioning, and 72 were treated at a Flu/Bu/TBI400 center, of whom 71 received Flu/Bu/TBI400. Importantly, no significant difference in transplant year, cytogenetic risk group, disease status at time of transplant, stem cell source, or graft versus host disease prophylaxis regimen was seen (Table 1). There were more patients with poor performance status in the Flu/Bu/TBI400 center (p = 0.004), and more ATG use in that centre (p < 0.001). In univariate analysis, there was no difference between centers in terms of OS and PFS (Figure 1). Similarly, in multivariable regression models there was no difference in either OS or PFS (Table 2). Cumulative incidence of non-relapse mortality was similar between groups (2 year point estimate of 12% in Cy/TBI1200 centers and 16.7% in the Flu/Bu/TBI400 centre, p=0.62). Cumulative incidence of relapse was lower in the Flu/Bu/TBI400 center (p = 0.05, 2 year point estimate of 31% in Cy/TBI centers and 18.5% in Flu/Bu/TBI 400 centers).
Discussion: In this recently treated cohort of adults ALL with minimal selection bias, we demonstrate that similar rates of overall and progression free survival can be achieved with conditioning regimens that contain low doses of TBI.
Daly:Sanofi-Genzyme: Other: Advisory Board. Seftel:Otsuka: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal