Abstract
BACKGROUND
Venetoclax(VEN), a once daily oral inhibitor of BCL2, has demonstrated high response rates and acceptable toxicity in patients with relapsed or refractory (R/R) CLL both as a single agent and in combination with the anti-CD20 monoclonal antibodies rituximab and obinutuzumab (formerly GA-101, G), where minimal residual disease (MRD) negative responses have been observed in the majority of patients. Ibrutinib (IBR), a once daily oral inhibitor of the Brutontyrosine kinase, likewise induces remissions in the majority of treated patients, but complete response (CR) is uncommon even after prolonged administration. Early genetic studies have demonstrated that BCL2 over-expression rescues BTK deficient XID murine B-cells from spontaneous apoptosis (J Immunol 1996), so we hypothesized that combination therapy would more efficiently achieve deep response endpoints. We report phase 1b results of a single-institution phase 1b/2 study of G, IBR, and VEN to characterize the safety and preliminary efficacy of the combination.
METHODS
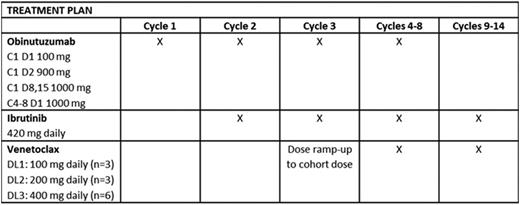
Patients with CLL relapsed after or refractory to ≥1 prior therapy and who required treatment were eligible. Enrolled patients had ECOG ≤1 and preserved end-organ function, including creatinine clearance ≥50 mL/min/m2. Patients with chronic viral hepatitis infection, uncontrolled autoimmunecytopenia, active Richter transformation, and known cysteine-481 BTK mutation or clinical disease progression during treatment with a cysteine-481-binding BTK inhibitor were excluded. G, IBR, and VEN were started sequentially over the first 3 of fourteen 28-day cycles as detailed in the table. To establish the safety of VEN in combination with OBIN and IBR, VEN dose was escalated in 3 x 3 cohorts (100, 200, 400 mg) to a maximum planned dose of 400 mg daily. Dose limiting toxicity (DLT) was defined during the third cycle. Risk assessment for VEN dose ramp-up was conducted according to US prescribing information. Adverse events were assessed and graded using CTCAE v4.03. Response assessment according to IWCLL 2008 criteria, including bone marrow biopsy with 4-colorimmunophenotyping of marrow and peripheral blood (PB) for MRD, occurs after cycles 8 and 14.
RESULTS
Twelve R/R patients have been treated in the phase 1b portion of the trial. Median age was 57 years (range: 42-70) and median prior therapies was 1 (range: 1-7). Baseline genetic risk features includedunmutatedIGHV in 11 (92%),del(17p) in 1 (8%), del(11q) in 8 (67%), and complex abnormal karyotype in 5 (42%) patients. Tumor lysis (TLS) risk was low in 1 (8%), medium in 7 (58%), and high in 4 (33%) patients at study entry. In general, observed toxicities for the combination were consistent with those reported for the single agents. DLTs were not observed at any VEN dose level, establishing VEN 400 mg daily as safe in combination with standard doses of G and IBR. The most common grade ≥3 adverse events (regardless of attribution) were neutropenia (50%), lymphopenia (33%),hypertension(25%), and fatigue (17%). Grade 1/2 adverse events occurring in over half the patients included bruising (all grade 1, 83%), infusion related reaction (75%), hypertension (67%), headache (67%), hyperuricemia (all grade 1, 75%), hypocalcemia (75%), and diarrhea (all grade 1, 67%), AST and/or ALT elevation (58%), and rash (50%). No cases of either clinical or laboratory TLS were observed. All patients remain on therapy and 6 have reached response assessment after completing 8 cycles of therapy. All 6 have achieved objective response: 5 PR, including 1 MRD-negative in PB (VEN 100) and 1 MRD-negative in both PB and marrow (VEN 100), and 1 CR with MRD-negative PB and marrow (VEN 200).
CONCLUSIONS
G, IBR, and VEN can be safely administered in combination at doses standard for the treatment of CLL. DLTs were not observed, establishing VEN 400 mg as the recommended phase 2 dose in combination with G and IBR. Adverse events were manageable and largely consistent with those reported in the single agent phase 2 studies. Objective responses, including MRD-negative responses, have been observed among all R/R patients from the first dose cohorts. Accrual continues to parallel phase 2 cohorts of R/R (n=25) and TN (n=25) patients. Updated phase 1b toxicity and response data will be presented.
Jones:Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Awan:Pharmacyclics: Consultancy; Novartis Oncology: Consultancy; Innate Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal