Abstract
Introduction: Allogeneic stem cell transplantation (SCT) remains a treatment option for patients with chronic myeloid leukemia (CML) that fail to respond to tyrosine kinase inhibitors (TKI). While the use of Imatinib seems to have no adverse impact on outcomes after transplant, little is known on the effects of prior use of second generation TKI (2GTKI). We present the results of a prospective non-interventional study (NIS) performed by the European Group for Blood and Marrow Transplantation (EBMT) of all consecutive allogeneic SCT for patients diagnosed with CML from 2009 to 2013.
Methods: A prospective follow up of pre and post-transplant data was carried out by the EBMT Leiden office, including TKI therapy data, MED-B and a specific MED-C post transplant data. A total of 94 EBMT centers from 32 countries included 437 patients.
Results: We present the results of the 383 patients that fulfilled all the inclusion criteria and had median follow-up of 37 months (1-77). The median age was 45 years (18-68) and 251 (65%) were males. Disease status at the start of 2GTKI was: First chronic phase - CP1 (123, 46%), Accelerated phase or >CP1 (67, 25%) and blast crisis (75, 28%). The choice of 2GTKI was: Dasatinib (155, 40%), Nilotinib (64, 17%) and a sequential combination of Dasatinib/Nilotinib with or without Bosutinib/Ponatinib (164, 43%). In addition, 29% of patients that received Dasatinib were in CP1 at the start of 2GTKI and at the time of SCT compared with 45% at the start and 40% at SCT for patients treated with Nilotinib. For patients that received both TKI in sequential combination, 63% were in CP1 at the start of 2GTKI but only 46% reached the SCT in CP1. Overall disease status at SCT was CP1 in 139 patients (38%), AP in 163 (45%) and BC in 59 (16%).
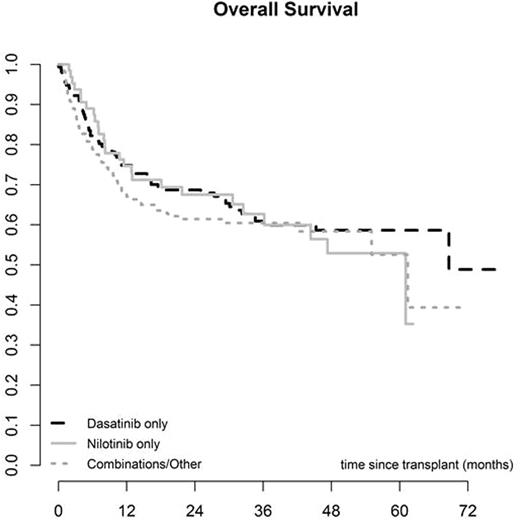
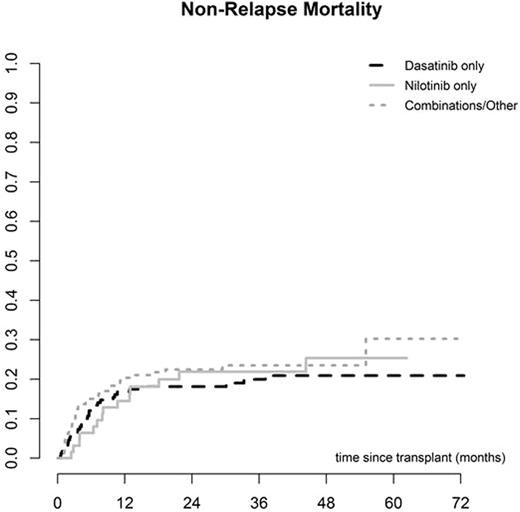
The median interval from diagnosis to SCT was 22 months (2 - 267) and the median interval between starting 2GTKI and SCT was 10 months (1 - 191). The donor was an HLA identical sibling in 130 cases (35%) and unrelated in 244 (65%). The majority of SCT were performed using PBSC (295, 77%), while 272 (71%) were myeloablative and 111 (29%) reduced intensity conditioning. The EBMT score was low (0-2) in 26 (7%), intermediate (3-4) in 216 (62%) and high (5-7) in 107 (31%). Primary graft failure (PGF) occurred in 10 (3%) cases, while the incidence of acute GVHD was 34% (95% CI 29-39) and chronic GVHD (CGVHD) was 60% at 5 years (95% CI 54-66). CGVHD occurred at a median of 5.7 months (3-61) post SCT. Other post SCT complications included veno-occusive disease of the liver (VOD) in 6 cases (2%) and severe infection in 195 (65%). There were no differences in post-transplant complications amongst the 3 different 2GTKI subgroups. Overall non-relapse mortality was 18% (95% CI 14-22) at 12 months and 24% (95% CI 19-29) at 5 years. Relapse incidence was 36% (95% CI 29-42), overall survival was 56% (95% CI 50-62) and relapse-free survival was 40% (95% CI 33-47) at 5 years. Overall survival was 67% (95% CI 59-75) at 5 years for patients in CP1. No differences in post-transplant outcomes were found amongst the 3 different 2GTKI subgroups. However, the EBMT score, performance status and disease stage at 2GTKI and at SCT were predictive of overall and progression-free survival.
Discussion: This prospective study demonstrates the feasibility of performing allogeneic SCT in CML patients previously treated with 2GTKI. The rate of post-transplant complications including graft failure, VOD, infections, GVHD and non-relapse mortality seems comparable to that of patients treated with Imatinib or TKI-naïve. We observed no differences between outcomes for patients receiving Dasatinib, Nilotinib or any other combination of 2GTKI (including Bosutinib and Ponatinib) pre-SCT. However, patients receiving Dasatinib were more likely to proceed to SCT in advanced phase than in CP1. Patients in CP1 have a very good overall survival despite prior treatment with 2GTKI. Of note, even after 2GTKI, the EBMT score remains a strong predictor of overall and disease-free survival for CML patients undergoing allogeneic SCT.
Schouten:Sanofi: Consultancy; Novartis: Consultancy. de Witte:Novartis: Honoraria, Research Funding; Celgene: Consultancy; Incyte: Consultancy. Kröger:Neovii: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Riemser: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal