Abstract
Aims: The impact of haematological malignancies (HM) on patients' health-related quality of life (HRQoL) is still not well understood. The aim of this study was to identify HRQoL issues and symptoms in patients with HM to be included in a new patient-reported outcome measure for use in routine clinical practice.
Methods: In a multicentre observational study carried out in the UK, adult patients with various HM, capable of reading English and give written informed consent were recruited from five hospitals in England and Wales. This qualitative study employed semi-structured face-to-face interviews with open-ended questions related to the impact of haematological malignancy and its treatment on HRQoL and symptoms. All the interviews were audio recorded and transcribed verbatim. Content analysis was carried out using the NVivo 11 qualitative analysis software. The themes and the sub-themes generated from the transcribed interviews were discussed during a 2-day "data definition" panel meeting by 2 hematologists, 1 patient research partner, 1 representative of a hematology patient organisation and 3 QoL research experts to select items for inclusion in the prototype instrument. These items will be further re-grouped and refined using cognitive debriefing, content validity and factor analysis.
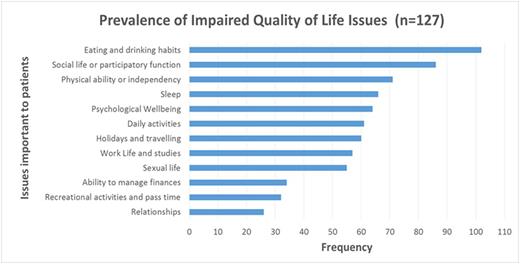
Results: 127 (male=75; mean age = 61.6 years; SD=15.1; median age 65.4 years; and age range =18-88 years) with mean duration of the HM of 3.7 years (SD=4.3; median=2.1 years; and range= 19 days-23 years) were recruited into the study. Diagnoses were: Acute Myeloid Leukaemia (18); Acute Lymphoid Leukaemia (7); Chronic Myeloid Leukaemia (12); Chronic Lymphatic Leukaemia (11); Aggressive Non-Hodgkin Lymphoma (16); Indolent Non-Hodgkin Lymphoma (14); Hodgkin Lymphoma (10); Multiple Myeloma (21); Myeloproliferative Neoplasm (10); and Myelodysplastic Syndrome (8). 383 items were reported by the patients under different themes and subthemes. 117 of these items were reported by more than 5% of the patients. 149 items were selected by the data definition panel to be included in the prototype instrument. The most prevalent QoL issues important to HM patients (Figure 1) were: 'eating and drinking habits (57 patients changed eating and drinking habits; 48 reported loss of appetite; 29 stopped drinking alcohol; and 11 reported increase in appetite); impaired social life and participatory function (86); impaired physical ability or independency (71); disturbed sleep (66); impaired psychological well-being (64); impaired daily activities (61); impaired ability to go on holidays or travelling (60); impaired work life & studies (57 ); impaired sexual life (55); impaired ability to manage finances (34); recreational activities and pastime (32); and relationships (26), from high to low prevalence, respectively. With respect to disease related symptoms, 102 issues were identified, the most prevalent being 'tiredness (65), feeling unwell (28), breathlessness (24), lack of energy (21), back pain (17) and weight loss (17)", from high to low prevalence respectively. Out of 124 treatment related symptoms identified, the most prevalent were: 'tiredness (73); feeling sick (36); lack of energy (20); taste disturbance (20); breathlessness (15); and diarrhoea (15)', from high to low prevalence respectively.
Conclusion: The findings of the qualitative and item generation phase clearly indicate that HMs affect patients' QoL significantly. However, in the absence of a validated measure for use in routine clinical practice, this is not captured in a systematic manner. Thus, this highlights the need for the development and validation of a new HM-specific PRO measure for use in such settings. Psychometric testing of the prototype instrument will be carried out to establish the measurement properties of the new HM-specific PRO measure.
Salek:EHA: Other: Educational and travel grant. Fielding:Amgen: Membership on an entity's Board of Directors or advisory committees; Baxalta: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Kell:Celgene: Honoraria; Novartis: Honoraria; Sunesis: Equity Ownership. Oliva:Pharma Companies: Honoraria; Italy: Speakers Bureau; Pharma Companies: Consultancy. Ionova:BMS: Research Funding; MSD: Other: lecturer bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal