Abstract
Background: Chemotherapy-induced anemia (CIA) is associated with a number of symptoms that negatively impact patients' quality of life. Management of these symptoms is essential for comprehensive patient care. However, with the exception of fatigue, a systematic examination of symptoms in patients with CIA is lacking. In order to better inform patient care, here we described the occurrence of a comprehensive list of CIA-related symptoms in patients with stage IV malignancies undergoing chemotherapy.
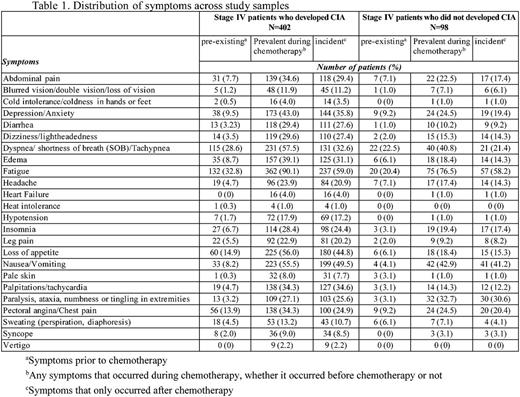
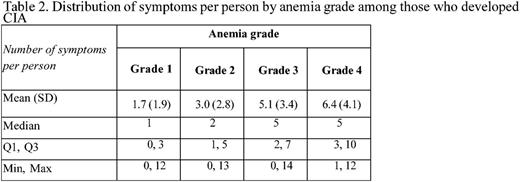
Methods: Patients diagnosed with incident non-Hodgkin lymphoma, breast and lung cancer at age 18 or older at Kaiser Permanente Southern California (KPSC) between 2010-2012 and initiated myelosuppressive chemotherapy prior to June 2013 were initially eligible for the study. Of these patients, those who did not have any hemoglobin measurement during the course of chemotherapy were excluded. Those who had the following conditions prior to chemotherapy were also excluded: less than 12 months KPSC membership, anemia, transfusion, radiation therapy or bone marrow transplant. CIA in this study was defined as hemoglobin <10 g/dl after the initiation of chemotherapy. Data on chemotherapy administration, hemoglobin measurements and other clinical characteristics were collected from KPSC's electronic medical records. Standardized chart review was performed to collect data on occurrence (based on physician documentation) of 24 pre-selected symptoms/signs for all patients with stage IV malignancies who developed CIA during the course of chemotherapy, and a random sample of patients with stage IV malignancies who did not develop CIA. The 24 symptoms/signs assessed are shown in Table 1. The prevalence of pre-existing symptoms (i.e., before chemotherapy), incident symptoms (those occurred only after chemotherapy) or prevalent symptoms (any symptoms during chemotherapy, whether they occurred prior to chemotherapy or not) across the study samples was described. The prevalence of each symptom and the distribution of number of symptoms per patient were also described by chemotherapy cycle and anemia grade. Anemia grade was defined following the Common Terminology Criteria for Adverse Events as grade 1: 10 g/dL to lower limit of normal (14 g/dL for men and 12 g/dL for women) ; grade 2: 8.0-9.9 g/dL; grade 3: 6.5-7.9 g/dL; and grade 4: <6.5 g/dL.
Results: A total of 402 patients who developed CIA and 98 patients without CIA were included. Among patients who developed CIA, fatigue was the most prevalent symptom during the course of chemotherapy, noted in 90% of the study sample. Dyspnea/shortness of breath (58%), nausea/vomiting (56%) and loss of appetite (56%) were documented in 50% or more of these patients (Table 1). Abdominal pain (35%), depression/anxiety (43%), dizziness/lightheadedness (30%), edema (39%), palpitations/tachycardia (34%), and pectoral angina/chest pain (34%) were documented in 30% or more of these patients. In patients without CIA, fatigue remained the most prevalent symptom (77% of the patients). However, only dyspnea/shortness of breath (41%), nausea/vomiting (43%) and paralysis/ataxia/tingling in extremities (33%) were noted in 30% or more of this study sample. There was not a clear increasing trend of the mean number of symptoms per patient across chemotherapy cycle. However, the mean number of symptoms per patient drastically increased as CIA grade increased (3 and 6 for grade 2 and 4 CIA, respectively, Table 2).
Conclusions: Our study provides greater insights into the burden of symptoms in patients with Stage IV malignancies who develop CIA. High grade CIA correlates with an increased symptom burden. These data support early treatment of CIA, before symptom burden rises steeply.
Chao:Amgen Inc.: Research Funding. Family:Amgen Inc.: Research Funding. Cannavale:Amgen Inc.: Research Funding. Pourmoussa:Amgen Inc.: Research Funding. Xu:Amgen Inc.: Research Funding. Bhakti:Amgen Inc.: Research Funding. Xu:Amgen Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal