Abstract
Introduction: DLBCL is the most common form of non-Hodgkin's lymphoma accounting for more than 30% of the new lymphoma cases in adults in the US.First-line treatment of patients with DLBCL typically includes immunochemotherapy with or without radiation therapy. Autologous HSCT is recommended in relapsed and refractory patients with chemosensitive disease. Allogeneic HSCT, a highly specialized procedure associated with intensive medical resources and costs, is generally considered for patients with refractory disease or who relapse following autologous HSCT.The objective of this study was to describe the short-term and long-term economic burden following an allogeneic HSCT procedure for adult patients with highest risk DLBCL.
Methods: Adult patients (≥18 years old) with DLBCL who underwent an allogeneic HSCT in a hospital setting were selected from two large US administrative claims databases (Q1 2006 to Q2 2015). Selected patients had continuous healthcare plan enrollment for ≥90 days prior to and ≥30 days after the date of the HSCT (index date). To characterize the short-term and long-term economic burden following allogeneic HSCT, healthcare resource utilization (HRU) and direct healthcare costs were measured and described over five study periods: 1) between the index date and the HSCT hospitalization discharge date, 2) during the first 100 days following the index date, and 3) during the first, 4) second, 5) third year following the index date. For each study period, HRU and costs were assessed among patients with continuous healthcare plan enrollment during the entire study period. Healthcare costs were measured from payers' perspective, reflecting the total amount reimbursed by the private payer and the amount covered by the coordination of benefits, excluding deductibles and copayments. Healthcare costs were adjusted for inflation and reported in 2015 USD.
Results: A total of 101 adult patients with DLBCL who received an allogeneic HSCT were identified. The average age was 54 years old at the time of the index date and 62% of patients were male. The median duration of the HSCT hospitalization was 24 days. The mean follow-up period after the index date was 1.3 years.
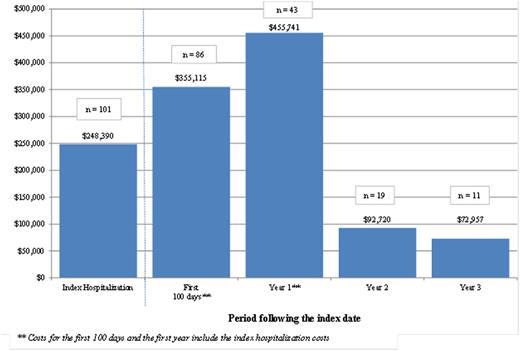
Results showed substantial HRU and costs following the allogeneic HSCT. The more intensive HRU and the highest healthcare costs were observed during the first year following the HSCT; patients had a mean of 2.5 inpatient admissions (including the hospitalization for the HSCT) for a total of 38 inpatients days and 68 days of outpatient services (apart from home care services), including 43 days with laboratory or imaging services. The mean total healthcare cost during the first year following the index date was $455,741 (median of $416,744; interquartile range from $301,344 to $575,662) - and the mean total healthcare cost during the hospitalization for the HSCT was $248,390 (median of $200,945; interquartile range from $156,908 to $281,279). Results also showed that although HRU and costs tend to decrease over time, they remain high even 3 years after the HSCT (Figure 1): during the third year following the index date, patients had a mean total healthcare cost of $72,957 (median of $76,749; interquartile range from $20,341 to $100,422), and mean of 27 days with outpatient services (apart from home care services), including 13 days with laboratory or imaging services.
Conclusion: Findings from this study showed that among adult patients with DLBCL, short-term and long-term economic burden following an allogeneic HSCT procedure is substantial.
Maziarz:Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Athersys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hao:Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Guerin:Analysis Group, Inc: Employment; Novartis Pharmaceuticals Corporation: Consultancy, Other: Annie Guerin is an employee of Analysis Group, which has received consultation fees from Novartis Pharmaceuticals Corporation. Gauthier:Analysis Group, Inc.: Employment; Novartis Pharmaceuticals Corporation: Consultancy, Other: Genevieve Gauthier is an employee of Analysis Group, which has received consultation fees from Novartis Pharmaceuticals Corporation. Gauthier-Loiselle:Analysis Group, Inc.: Employment; Novartis Pharmaceuticals Corporation: Other: Marjolaine Gauthier-Loiselle is an employee of Analysis Group, which has received consultation fees from Novartis Pharmaceuticals Corporation. Thomas:Novartis Pharmaceuticals Corporation: Employment. Eldjerou:Novartis Pharmaceuticals Corporation: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal