Abstract
Background: There is a scarcity of data about healthcare resource utilization (HRU), costs, and treatment patterns of patients (pts) with newly diagnosed AML, despite the prolonged hospitalizations associated with induction and consolidation chemotherapy. The initial decision whether to treat with intensive induction chemotherapy depends, primarily, on pt age, comorbidities, and performance status. For pts deemed eligible for induction chemotherapy, treatment generally consists of induction with an intensive chemotherapy regimen (e.g., cytarabine + anthracycline "7+3") and post-induction therapy (consolidation) for those who achieve a complete remission. Post-induction therapy may consist of repeated rounds of further cytotoxic chemotherapy or allogeneic HSCT.
Methods: Adult pts with newly diagnosed AML who received induction chemotherapy in an inpatient (IP) setting within 14 days of diagnosis were identified from a US administrative claims database (2006-2015). Pts with acute promyelocytic leukemia (APL) or in clinical trials were excluded. Outcomes were analyzed between the beginning of the induction episode and an HSCT, death (IP discharge status of death), end of health plan enrollment/ data availability, or 180 days after the discharge date of the induction episode, whichever occurred first (i.e., observation period). Induction and consolidation episodes were identified using diagnosis related group (DRG) codes for chemotherapy with acute leukemia (DRG 837, 838, or 839) or procedure/drug codes for specified/unspecified chemotherapy for AML. Outcomes included treatment patterns (setting and duration of AML treatment episodes, and time between episodes), HRU (IP admissions, HSCT), and costs (USD 2015, amount reimbursed by private payers, coordination of benefits, and beneficiaries contributions [deductibles and copayments]). AML treatment costs are reported during induction and consolidation episodes and IP costs are reported between episodes.
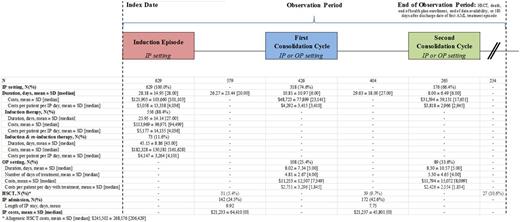
Results: A total of 631 pts (mean age = 56 years; 48% female) met the sample selection criteria. Among these, during the 12-month period preceding the index date, 18% had a diagnosis for MDS, 15% had a diagnosis for a malignancy other than lymphoid/hematopoietic neoplasms (mainly breast or skin malignancies), 14% received chemotherapy, 1% received radiation therapy, and the average Charlson comorbidity index score was 2.3.
During the observation period, 2 out of 631 pts had a HSCT during the IP stay of the induction therapy (and were excluded from the cost analysis). A total of 73 (12%) pts had initial induction and subsequent re-induction therapy during the same IP episode. The median duration of IP episodes for pts who required initial induction therapy only was 27 days, the mean total healthcare cost was $113,969, and the mean cost per day was $5,177. The median duration of IP episodes for pts who required induction and re-induction therapy was 43 days, the mean total healthcare cost was $182,328, and the mean cost per day was $4,147.
Among pts with post-induction treatment episodes, the first consolidation cycle occurred, on average, 26 days after the discharge date of the induction episode; 75% was in an IP setting, and 25% in an OP setting. In the IP setting, the median duration of the first cycle was 6 days, the mean total healthcare cost was $48,723, and the mean cost per day was $4,292. In the OP setting, the median duration of the first cycle was 4 days of treatment over 5 days, the mean total healthcare cost was $11,253, and the mean cost per day of treatment was $2,751.
A proportion of 25% of pts had an IP admission between the end of the induction episode and the first consolidation cycle, and 43% between the first and second consolidation cycles. In addition, during the observation period, a total of 97 (15%) pts had an allogeneic HSCT; 31 (5%) after the induction episode, 39 (6%) after the first consolidation cycle, and 27 (4%) after the second consolidation cycle. (Table)
Conclusions: This is the first exploratory study reporting treatment patterns, HRU, and costs of AML management. Despite lack of granular information on the type of treatment administered, this study describes the setting, duration, and HRU and costs associated with induction and consolidation therapy for AML. Study findings suggest that in a sample of commercially insured pts with newly diagnosed AML, there is substantial heterogeneity in the management and costs of AML.
Stein:Agios Pharmaceuticals: Other: Advisory Board, Research Funding; Seattle Genetics: Research Funding; Celgene: Other: Advisory Board, Research Funding; Novartis: Consultancy. Latremouille-Viau:Analysis Group: Employment; Novartis: Other: Author is an employee of Analysis Group which has received consulting fees from Novartis. Guerin:Novartis: Other: I am an employee of Analysis Group, Inc., which has received consulting fees from Novartis for the conduct of this study; Analysis Group, Inc.: Employment. Shi:Novartis: Other: I am an employee of Analysis Group, Inc., which has received consulting fees from Novartis for the conduct of this study; Analysis Group, Inc.: Employment. Gagnon-Sanschagrin:Analysis Group, Inc: Employment; Novartis: Other: Author is an employee of Analysis Group which has received consulting fees from Novartis. Bonifacio:Novartis: Employment. Joseph:Novartis: Employment; Amgen: Other: Stocks/stock option; Pfizer: Other: Stock/stock option.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal