Abstract
Introduction: Multiple myeloma (MM) is a disease of the elderly with a median age at diagnosis of 70 years. Autologous stem cell transplantation (ASCT) is a preferred treatment for younger patients but is also an option for older adults (i.e., patients >65 years) with good performance status. A previous SEER-Medicare study of MM patients diagnosed from 2000-2007 found that 5.8% underwent ASCT (Winn, et al. JNCI, 2015). The availability of ASCTs for older adults with MM has increased in the past decade due to improvement in supportive care and Medicare coverage approval. Apart from age and medical eligibility, several patient and contextual factors, such as comorbidity, may influence the receipt of ASCT in the MM population. There is limited information on the determinants of receipt of ASCT in older adults over the past decade in the US.
Objective: To identify ASCT recipients among a cohort of elderly individuals with MM in order to determine characteristics associated with receiving ASCT. Specifically, this study identifies patient and contextual factors associated with the receipt of ASCT.
Methods: This retrospective cohort study used Surveillance, Epidemiology, and End Results (SEER) registry and linked Medicare claims (SEER-Medicare) data. We identified individuals aged 66 and above, with an incident diagnosis of MM between 2007 and 2011 as well as claims data from 2006 to 2012. We required continuous enrollment in Medicare Parts A and B 12 months prior to and including the month of diagnosis and six months post-diagnosis. We required continuous Part D enrollment two months pre- and six months post-diagnosis. Patients were followed until death or censoring due to non-continuous Parts A and B enrollment after six months. ICD-9 and HCPCS codes were used to identify ASCT. Charlson Comorbidity Index (CCI) was used to measure the number of comorbid conditions at the time of MM diagnosis using claims one year prior to diagnosis. Student's t-test and Chi-square test of proportions were used to compare those who received ASCT to those who did not based on patient-level factors (i.e., age, gender, race, comorbidity status), geographic regions (i.e., Northeast, Midwest, West and South), and over time (diagnosed in 2007-2009 vs. diagnosed in 2010 to 2011). We also measured the time to ASCT for those who underwent ASCT.
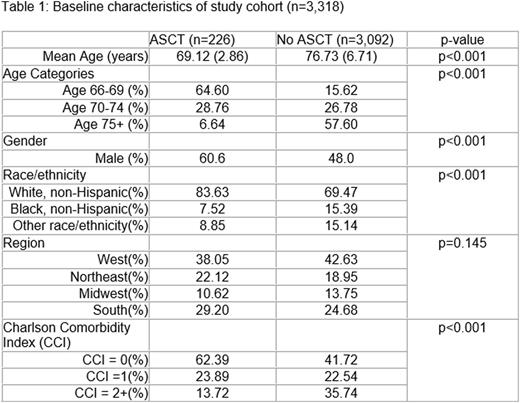
Results: Among 3,318 individuals with MM who met our inclusion criteria, 226 (6.8%) underwent ASCT during the follow-up period. ASCT recipients were younger, more likely to be male, white non-Hispanic, and have fewer comorbid conditions (Table 1). The median time from MM diagnosis to ASCT was 278 days. The rate of ASCT among recipients aged 66-69 was 23.2%, 7.3% among recipients aged 70-74, and 0.84% among those aged 75+ (p<0.001). The rate of ASCT was higher among males (8.5%) than females (5.2%) (p<0.001). Rates of ASCT were higher among those who were non-Hispanic white (8.1%), compared to those who were non-Hispanic black (3.5%) or of another race/ethnicity (4.1%) (p<0.001). Among those with CCI=0, 9.9% underwent ASCT, while 7.2% of those with CCI=1 underwent ASCT and 2.7% of those with CCI>1 underwent ASCT (p<0.001). Rates of ASCT were similar across the geographic regions (p=0.15). Of those who were diagnosed in 2007-2009, 6.0% received transplant, while 7.8% of those who were diagnosed in 2010-2011 received transplant (p=0.04).
Conclusion: ASCT is performed in less than 1 in 10 patients aged 66 and older. A greater proportion of ASCT recipients were non-Hispanic white, male, diagnosed at a younger age, and had a lower comorbidity burden compared to non-transplant patients. Comparing the pre-2007 estimate from the aforementioned study to our early (2007-09) and late (2010-11) period estimates, our results illustrate an upward trend in ASCT over the past decade. We were unable to identify smoldering MM patients who would not be candidates for ASCT. This may bias our estimates downward. The aforementioned previous study found that 62% of recipients were 66-69, 32% of recipients were 70-74, while 6% were 75+ (Winn, et al. JNCI, 2015). These rates did not differ greatly from our findings indicating that the age distribution of ASCT recipients has remained stable over a 10 year period of observation. Future studies should investigate the implications of these differences for post-transplant outcomes among older MM patients.
Goto:Novartis AG: Research Funding. Onukwugha:Takeda: Research Funding; IMPAQ International: Honoraria; Bayer Healthcare: Research Funding. Seal:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Romanus:Takeda: Employment. Yong:Takeda: Employment. Slejko:Takeda: Research Funding; PhRMA: Research Funding; National Pharmaceutical Council: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal