Abstract
Background: Allo-SCT is a curative option for patients with AML and MDS. It is unclear whether there is an upper age limit for Allo-SCT. CIBMTR showed similar outcomes for patients older than 65 years after Allo-SCT (McClune et al J Clin Oncol 28:1878-1887). A single center report of 54 patients (70 - 75 years) established the feasibility of Allo-SCT in patients over age 70 (Brunner et al Biol Blood Marrow Transplant 19:1374-1380). There is no published data on Allo-SCT in patients older than 75 years.
Methods: Retrospective analysis was performed on all patients with diagnosis of AML or MDS who underwent an Allo-SCT after their 75th birthday at UMass Memorial Medical Center on an IRB approved protocol.
Results: 14 patients were identified from the database. Median age at transplant was 78.4 years (range 75.2 - 83.6). Diagnosis was AML (N=10) and MDS (N=4). Disease status at SCT for AML patients was CR1 (N=6), ≥ CR2 (N=2) and relapse (N=2). Source of stem cell was peripheral blood (PB) (N=11) and cord blood (CB) (N=3). Median time from diagnosis to SCT was 7 months (range 3 - 98). Karnofsky score (KS) at SCT was 90 (N=5) and 80 (N=9). Three patients had prior autologous SCT. Source of stem cells was peripheral blood (PB) (N=11) and cord blood (CB) (N=3). Donors were mostly unrelated (N=13) with one being mismatched related (N=1). For PB recipients the HLA matching was 10/10 in 7 patients and 9-11/12 in 4 patients. CMV serostatus was neg/neg (N=2), pos/pos(N=4) and pos/neg (N=7). ABO mismatch was major (N=1) and minor (N=6).
Conditioning regimen was reduced intensity in 13/14 recipients. All three CB recipients received Melphalan (Mel) based conditioning (Thio/Flu/Mel 100-140mg/m2). Conditioning for PB recipients was Busulfan(Bu) based (N=6) and Mel (dose 50 - 140mg/m2)based (N=5). Graft versus Host Disease (gvhd) prophylaxis was Tacrolimus (Tac) with Mycophenolate mofetil (MMF) for all CB-SCT recipients Flu/Bu-2 recipients. All Mel based PB-SCT recipients received post-transplant Cyclophosphamide (days 3, 4) with Sirolimus. HLA mismatched patients also receive Tac. 11/14 patients received ATG.
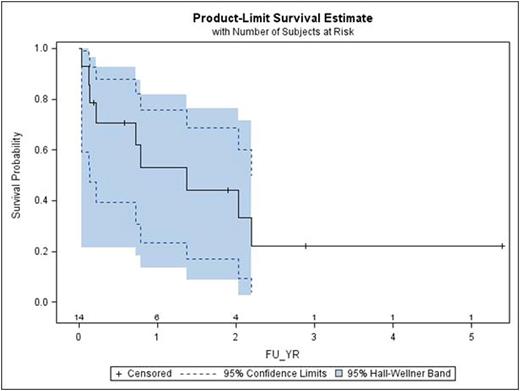
All PB recipients engrafted their neutrophils at a median of 16 days (range 13-21). 10/11 patients engrafted their platelets at a median of 18 days (range 0-32). One CB recipient died on day 10 before engrafting. Other two CB recipients engrafted their neutrophils on day 14 and platelets on days 33 &37. Day 100, 1- year and 5 - year overall survival for the entire cohort was 70.7% (95% CI 39.4-87.9), 53% (95% CI 23.3-75.9) and 22.1% (95% CI 3.8 -49.9) respectively. Two patients developed grade III and 3 patients developed grade II acute gvhd. Chronic (limited) gvhd was seen in one patient only. 9 patients died at a median of 264 days (range 10 - 802) post SCT. There were no deaths due to disease progression. Three patient are currently surviving beyond 2 years. Two of these patients are immune suppression free, gvhd free and live alone with a KS of 100. 3 out of 4 patients over age 80 years survived more than 2 years.
Conclusion: Allo-SCT is feasible in selected patients over the age of 75.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal