Abstract
Purpose: Relapse after allogeneic hematopoietic SCT (allo-SCT) for AML or myelodysplastic syndrome (MDS) remains the main cause of treatment failure and is associated with a very poor prognosis and a short survival despite the type of the salvage therapy. Options include reductions of immunosuppressive therapy, chemotherapy with or without infusion of donor lymphocytes (DLI) or second allo-SCT, but results remain disappointing. Other studies (C Jabbour et al, and Craddock Charlie et al) demonstrated the efficacy and safety of 5-azacytidine (Vidaza) maintenance post Allo-SCT.
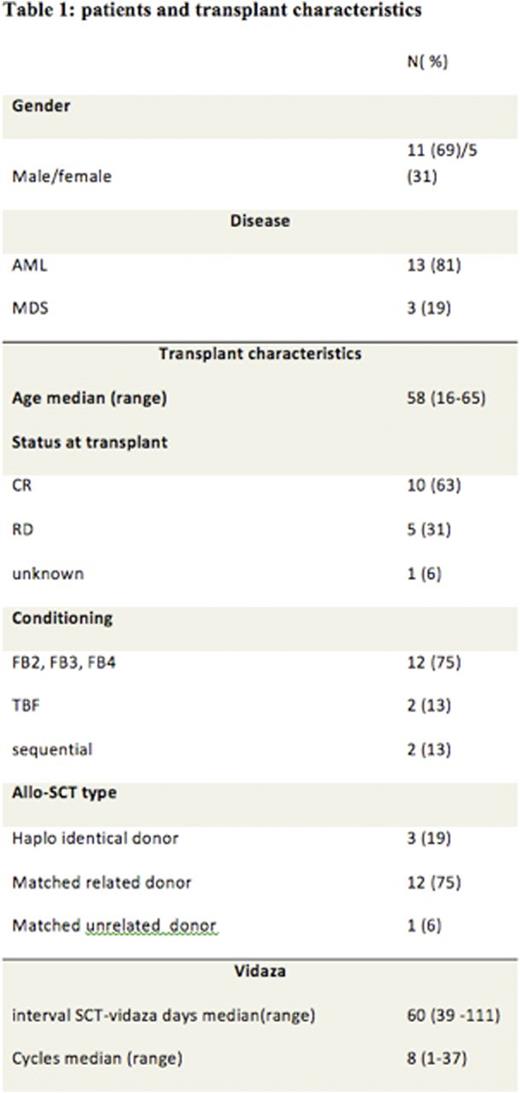
Patients and Methods: The aim of our observational study was to evaluate the anti-leukemic effect of 5-azacytidine (Vidaza) as post-transplantation prophylactic therapy to prevent relapse in patients with high risk AML or MDS in our center. We identified 16 patients, 13 AML and 3 MDS. Twelve patients (75%) received a risk-adapted intensity of busulfan at 130mg/m2/day for either 2, 3 or 4 days, with a fixed dose of fludarabine (30mg/m2/day for 5 days), and thymoglobulin (2.5mg/kg/day for 2 days). Our algorithm was based on age, co-morbidity (ies) and disease risk.
Twelve patients (75%) received peripheral blood stem cells (PBSC) from HLA identical siblings, 3 patients (19%) from haplo-identical donor and 1 patient from matched unrelated donor. Azacitidine was started after a median of 60 days post-transplant with a reduced dose of 32 mg/m2/day for 5 days monthly for 5 years. None of the patients experienced graft rejection. Patient and transplant characteristics are outlined in Table 1.
Results: Nine patients (56%) had either a monosomal karyotype or a complex karyotype. Patients received a median number of 8 cycles (1-37) of vidaza. None of our patients stopped definitively vidaza for toxicity reasons. Three patients (19%) discontinued their treatment: one for 3 months because of pneumonia, one for acute GvHD grade II then resumed Azacitidine therapy after 6 months, the third stopped for 2 weeks because of neutropenia. After a median follow-up of 10 months (1-56) from allo-SCT, 13 patients (81%) still alive and 3 patients died; one because of CMV infection, one from disease progression and one from cardiac infarct. At the last follow up 13 patients (81%) remain in complete remission.
Conclusions: Azacitidine prophylactic maintenance therapy appears to reduce the risk of relapse by helping consolidate post-allograft responses in high risk AML or MDS. The toxicity related to this dose of azacitidine was very low. Our findings require validation in a large prospective multicenter randomized study.
Legend: AML, Acute Myeloid Leukemia; MDS, Myelodysplastic Syndrome; CR, Complete Remission; FB2 Fludarabine 2 days Busulfan,;FB3 Fludarabine 3 days Busulfan; FB4 Fludarabine 4 days Busulfan; TBF Fludarabine Busulfan.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal