Abstract
Background:
Peripheral T-cell lymphomas (PTCL) are a rare and heterogeneous group of non-Hodgkin lymphomas (NHLs) that accounts for approximately 10% of all aggressive NHLs in Western countries. The optimal management remains unclear, however, given the poor outcome, allogeneic transplant (allo-SCT) has been integrated into the front-line treatment for some rare extranodal subtypes as well in relapsed/refractory setting. We report our provincial experience of the outcome of patients with PTCL who have undergone allo-SCT at the British Columbia Cancer Agency (BCCA).
Methods:
The Leukemia/BMT Program of British Columbia database and the BCCA Lymphoid Cancer Database were searched to identify all patients diagnosed with PTCL who have undergone allo-SCT between November 1990 and January 2016. Overall survival and relapse free survival were estimated using the Kaplan-Meier method.
Results:
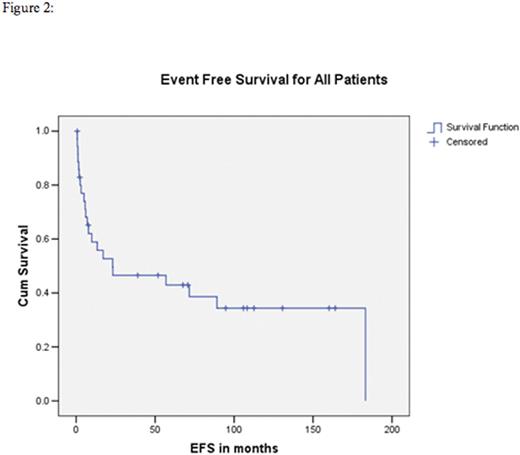
We identified 36 cases of PTCL patients who have undergone allogeneic transplant from a median time of 11 months from primary diagnosis (range 4-64) with the following clinical features: median age at transplant was 45 years (range 16-58 years); 24 (67%) were male; 32 (89%) patients had advanced stage disease; 22 (61%) had B-symptoms at diagnosis. Bone marrow involvement detected in 13/34 (38%) patients. Histological diagnosis based on the WHO 2008 classification were: Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) n=15 (42%); anaplastic large cell lymphoma (ALCL) n=7 (19%) out of which three were ALK positive, angioimmunoblastic T-cell lymphoma (AITL) n=6 (17%), hepatosplenic T-cell lymphoma n=5 (14%), enteropathy associated T-cell lymphoma type I n=1, advanced primary cutaneous gamma-delta T-cell lymphoma n=1 and Sezary syndrome n=1. Ten patients (28%) underwent allo-SCT as part of planned primary therapy after achieving their first remission [6 were in complete remission 1 (CR1) and 4 were in partial remission 1 (PR1)], whereas 26 patients (72%) underwent allo-SCT for relapsed/refractory disease. CHOP was administered in 19 patients (53%) as the primary therapy. 17 (47%) patients received alternative chemotherapy regimen due to patient and/or physician preference. The clinical status at the time of transplantation was CR in 12 patients (33%), relapsed sensitive disease n=10 (28%), relapsed untreated n=5 (14%), partial remission (PR) in 5 (14%), primary progressive disease n=3 (8%) and relapsed resistant disease n=1 (3%).
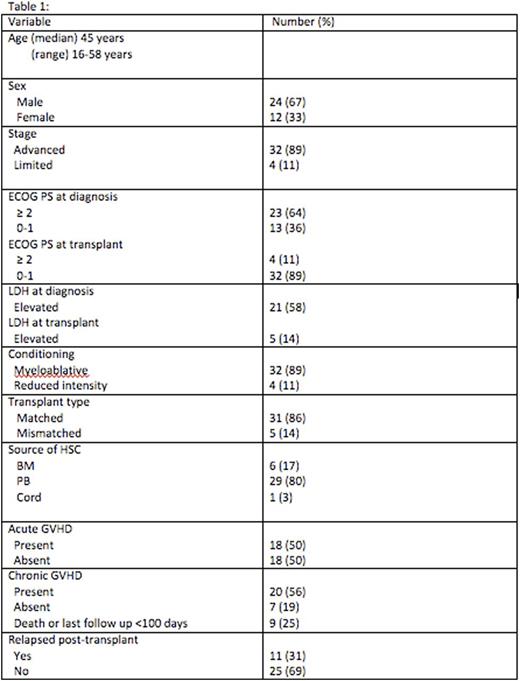
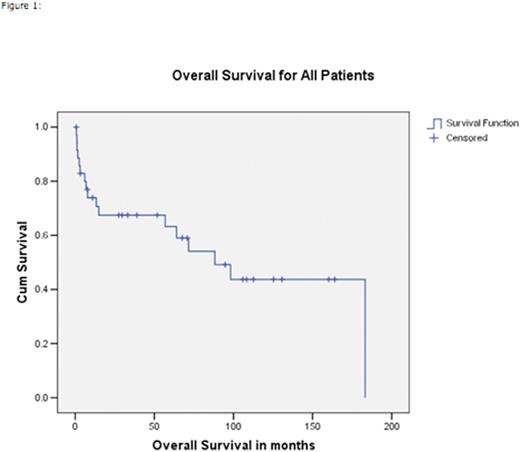
Thirty two patients (89%) underwent myeloablative conditioning, 4 (11%) underwent reduced intensity conditioning (RIC). The conditioning regimens included: cyclophosphamide/TBI n=24 (67%), busulfan/cyclophosphamide n=5 (14%), fludarabine containing reduced intensity n=4 (11%), and other regimens n= 3 (8%). With a median follow-up of alive patients from the time of allo-SCT of 69 months (range 1-186 months). At last follow-up, 17 (47%) patients have died, 6 from disease relapse, 5 from graft vs host disease (GVHD), 2 from regimen related toxicity and 4 from other causes. Nineteen patients (53%) still alive at last follow up post-transplant of which 14 (39%) still in remission. The 5-year event free survival (EFS) and overall survival (OS) from the time of allo-SCT of all patients were 43% and 63%, respectively. For PTCL-NOS the 5-year EFS and OS were 52% and 69%, respectively. Table 1 summarizes the patients' characteristics. Figures 1 and 2 shows the Kaplan-Meier curves for OS and EFS respectively.
Conclusion:
Allo-SCT can be effective strategy in select patients with relapsed/refractory PTCL and those with high risk histologies in the upfront setting PTCL with an acceptable toxicity.
Toze:Roche Canada: Research Funding. Sehn:roche/genentech: Consultancy, Honoraria; amgen: Consultancy, Honoraria; seattle genetics: Consultancy, Honoraria; abbvie: Consultancy, Honoraria; TG therapeutics: Consultancy, Honoraria; celgene: Consultancy, Honoraria; lundbeck: Consultancy, Honoraria; janssen: Consultancy, Honoraria. Scott:NanoString Technologies: Patents & Royalties: named inventor on a patent for molecular subtyping of DLBCL that has been licensed to NanoString Technologies. Villa:Lundbeck: Honoraria; Roche: Honoraria, Research Funding; Celgene: Honoraria. Connors:NanoString Technologies: Research Funding; F Hoffmann-La Roche: Research Funding; Bristol Myers Squib: Research Funding; Seattle Genetics: Research Funding; Millennium Takeda: Research Funding. Song:Janssen: Honoraria; Otsuka: Honoraria; Celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal