Abstract
Background: A second (salvage) ASCT has frequently been offered to MM patients with relapsed disease who experience benefit from the first procedure. We have previously reported that pts undergoing a salvage ASCT in the era of VAD or thalidomide (thal) have a median progression-free survival (PFS) of 19 months (mos). The best results were observed in pts who experienced ≥ 2 year benefit after their first ASCT (Jimenez-Zepeda VH et al. Biol Blood Marrow Transplant 2012; 18: 773-9). However, the utility of this approach after the introduction of novel chemotherapeutic agents--such as bortezomib (BTZ)--remains unclear. Initially, provincial funding for BTZ in Ontario was provided only for relapsed disease. However, in 2007, the combination of either BTZ + dexamethasone (BTZ-dex) or cyclophosphamide, BTZ + dex (CyBorD) was adopted as the standard induction regimen for newly diagnosed pts before ASCT performed as part of first-line therapy. We now examine the results of salvage ASCT in our centre after the availability of BTZ.
Methods: We used the Princess Margaret Myeloma Database to identify and characterize patients with relapsed MM who had received a bortezomib (BTZ)-based regimen for remission induction prior to their first ASCT or for re-induction before salvage ASCT. A retrospective chart review was performed to investigate the PFS and overall survival (OS) outcomes of these pts.
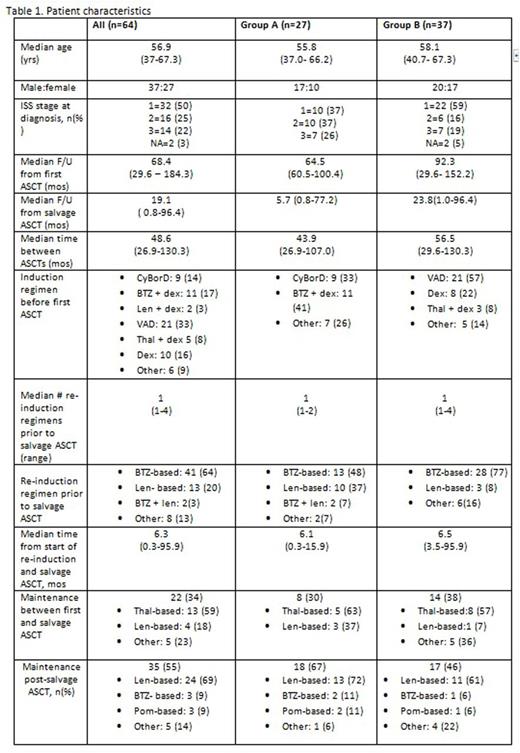
Results: Between 01/2005 and 07/2015, 64 pts with MM who had previously received BTZ-based therapies underwent salvage ASCT for relapsed disease at our centre (Table 1). Median age was 56.9 yrs (range 37-67.3); 37 (58%) were male. ISS stage was 1 in 32 (50%), 2 in 16 (25%), 3 in 14 (22%) and NA in 2 (3%). The median interval between first and salvage ASCT for all pts was 48.6 mos (range 26.9-130.3), reflecting our policy of preferentially offering salvage ASCT to pts with at least a 2-yr benefit from the first transplant; the median time between re- induction therapy and salvage ASCT was 6.3 mos (range 0.3-95.9). Group A pts (n=27) had received BTZ-based therapy before their first ASCT; 48% of these also received BTZ-based regimens again prior to salvage ASCT. Pts in Group B (n=37) received BTZ-based regimens before the salvage transplant only, while induction therapy before the first ASCT consisted of VAD (21), dex alone (8), thal + dex or other regimens (5). Twenty-two (34%) of the pts received maintenance therapy between the first and salvage ASCT (most often thal-based), while 35 (55%) of the pts received maintenance therapy following salvage ASCT (most frequently lenalidomide [len]-based).
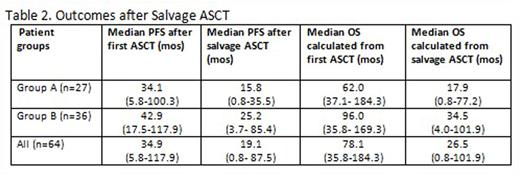
The survival outcomes are summarized in Table 2. Median duration of follow-up (F/U) following salvage ASCT was 19.1 mos (range 0.8-96.4). One patient (1.6%) died several days following salvage ASCT. No other transplant-related mortality occurred. The median PFS following salvage ASCT was 19.1 mos (range 0.8- 87.5) with a median OS of 26.5 mos (range 0.8-101.9) in all pts. The median PFS after salvage ASCT was 15.8 mos for Group A and 25.2 mos for Group B pts.
Conclusions: Even in the era of novel agents, salvage ASCT may provide PFS benefit to pts with relapsed MM who were previously treated with a BTZ-based regimen. However, the details of the optimal approach in this setting are not certain, including the impact of maintenance therapy given after the first and/or salvage ASCT. We are performing additional analyses of this population to try to identify factors associated with the best outcomes.
Kukreti:Celgene: Honoraria; Lundbeck: Honoraria; Amgen: Honoraria. Prica:Janssen: Honoraria. Tiedemann:Novartis: Honoraria; Celgene: Honoraria; Takeda Oncology: Honoraria; BMS Canada: Honoraria; Amgen: Honoraria; Janssen: Honoraria. Trudel:Celgene: Honoraria; Novartis: Honoraria; Glaxo Smith Kline: Honoraria, Research Funding; Oncoethix: Research Funding. Reece:Merck: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal