Abstract
Introduction: Post-remission therapy in acute myeloid leukemia (AML) is mainly guided by cytogenetic and molecular risks, patient age and performance status. Favorable AML is conventionally treated with induction chemotherapy followed by 3-4 cycles of consolidation. Despite the clear benefit of the graft-versus-leukemia effect, allogeneic stem cell transplantation (alloSCT) is mainly reserved for fit patients with genetic intermediate-poor risk due to its toxicity. Autologous SCT (ASCT) performed at remission allows intensification of the anti-leukemic effect by the administration of high-dose therapy without significant non-relapse mortality (NRM), as compared to chemotherapy. This approach may be particularly appealing in the setting of AML with favorable genetic risk where alloSCT is not the standard of care. The aim of the current study was to retrospectively evaluate the outcome in a large, single-center cohort of favorable-risk AML patients based on post-remission treatment applied.
Methods: Consecutive AML patients with the core-binding factor (CBF) translocations or mutations of NPM1 gene/unmutated FLT-ITD treated at the Rambam Medical Center, Haifa, Israel, between the years 2003 and 2015 were included in the analysis. Patients in complete remission (CR) post induction were planned for 2 cycles of consolidation with high-dose ARA-C (HiDAC) followed by ASCT. Patients in whom insurance coverage was denied continued with 3-4 cycles of HiDAC consolidation. Additionally, patients with evidence of post-induction residual disease, as determined by PCR, with a complex karyotype at presentation (in addition to CBF) or diagnosis of secondary AML were referred to alloSCT in CR1 and are also reported here.
Results: Seventy nine patients with favorable AML with a median age of 47 [range 18-73] years were identified. Thirty eight (48.1%) patients had mutated NPM1 /unmutated FLT-ITD, 18 (22.8%) patients were with t (8:21) and 23 (29.1%) patients with inv(16). Forty patients (50.6%) received chemotherapy alone, 26 patients (32.9%) received chemotherapy and ASCT and 13 (16.4%) underwent alloSCT. There was no difference between the 3 treatment groups in regard to: response to induction, daunorubicin dose in induction, and need for reinduction. However, age was significantly higher in the chemotherapy group compared with ASCT and allo SCT groups.
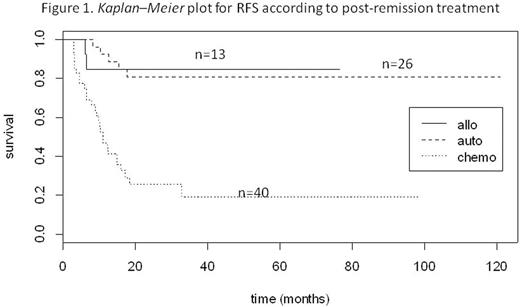
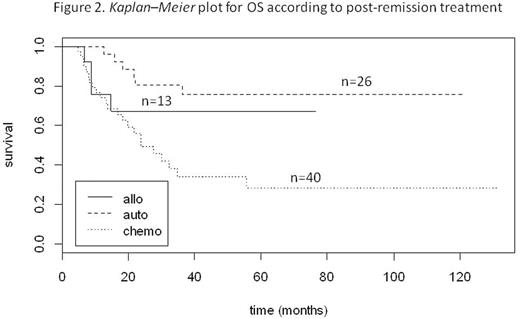
Two-year relapse-free survival (RFS) was 25%, 81%, and 85% for the chemo, ASCT and alloSCT, respectively (p < 0.00001) (Fig 1). Median RFS was 10.9 months for the chemotherapy group, while it was not reached (NR) either for the ASCT or the allo SCT group.Two-year overall survival (OS) was 49%, 80%, and 67% for the chemo, ASCT and alloSCT groups, respectively (p = 0.00269)(Fig 2). Median OS was 23.6 months for the chemotherapy group, while it was not reached for the ASCT group (NR to NR) and the alloSCT group. NRM at 3 months was 4/40 (10.0%), 0/26 (0%) and 2/13 (15.4%) in the chemo, ASCT and alloSCT groups, respectively. Relapse rate was 70.0% in the chemo group, 15.4% in the ASCT group and 19.2% in the alloSCT group (p < 0.0001). In the ASCT group, among the relapsing patients, 80% were referred to alloSCT. With a median follow-up of 28.6 months (range 4.3-131.4), 45 patients are alive and in CR. In univariate analysis, age and post-induction therapy, which included transplantation (ASCT or alloSCT), were found to be associated with higher OS and RFS.
Conclusions: RFS and OS were significantly superior in AML patients with favorable cytogenetics receiving post-induction high-dose therapy with either ASCT or alloSCT. This was achieved with no transplant related mortality or increased toxicity among patients receiving ASCT compared with the chemotherapy group. These data from a single institution support the recent resurgence of interest in ASCT for AML and emphasize the potent anti-leukemic efficacy and minimal toxicity of this modality for patients who are not candidates for an allo SCT. After these decades, it may be time to once again conduct large prospective randomized trials of ASCT versus chemotherapy alone.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal