Abstract
Background:
Myelofibrosis (MF) is a myeloproliferative neoplasm that is characterized by significant scar tissue and fibrosis in the bone marrow, enlarged spleen and/or liver from extramedullary hematopoiesis, and may include significant constitutional symptoms such as bone pain, night sweats, pruritis, and cachexia. The only curative therapy is allogeneic stem cell transplant. Although the symptom burden has been explored in the literature, the impact of hematopoietic stem cell transplant (HCST) on QoL in patients with MF has not been evaluated. We sought to longitudinally describe QoL in patients undergoing HCST for MF.
Methods:
We prospectively followed patients undergoing HSCT for MF. We assessed symptoms, functioning, and QoL using the FACT-BMT and MPN-SAF total symptom score (TSS) pre-transplant and at day 30, day 100 and one year post-transplant. Scores at the post-transplant time points were compared with baseline scores by paired t-tests. Pearson correlations between FACT-BMT and MPN-SAF TSS questionnaires were also computed.
Results:
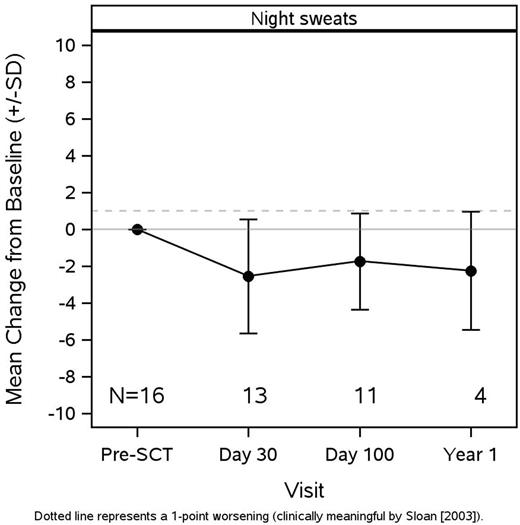
16 patients were enrolled [median age 64.0 (49-69) years; 13 (81%) male; 13 (81%) Caucasian], two did not have day 30 data as they died prior to then or did not go to transplant. Fourteen patients had day 30 information, 11 had day 100 information, and only 4 had one year information. Of the 14 who had day 30 information, 6 patients died within the first year, two from treatment related mortality and four from relapse. One patient had intermediate-1 risk, the remainder of the patients were intermediate-2 or high risk. All patients had RIC conditioning. Mean MPN-SAF TSS score was 28.1 (SD=14.2) and FACT-BMT total score was 99.8 (SD=17.4) at baseline. FACT-BMT and MPN-SAF TSS at baseline were inversely correlated; lower symptom score was associated with higher QoL (r=-0.62; p=0.01). FACT-BMT at day 30 was lower (mean change: -12.5, SD=16.7; p=0.03). Two MF-specific symptoms showed improvement that reached statistical significance compared to baseline: night sweats mean improvement day 30, 2.5 (SD=3.1; p=0.01) and mean improvement day 100, 1.7 (SD=2.6; p=0.05, Figure 1); headache mean improvement day 100, 1.5 (SD=1.9) p=0.02. In general, scores showed a worsening at day 30, improvement at day 100 and stability at one year. The MPN-SAF TSS worsened at day 30 (6 points) and improved by day 100 (4.5 points). Changes that showed improvement at day 100 include Brief Fatigue Inventory (BFI) with a mean improvement of 1.2 points and concentration (1 point). Of the four surveys that were collected at one year, a modest decline was noted in BFI (1.5 points), inactivity (1.5 points) and cough (3 points). However improvements were noted in night sweats (2.25 points), abdominal discomfort (1 point), insomnia (1.75 points), bone pain (1 point).
Discussion:
This is the first study to evaluate serially the QoL and symptom burden of patients who underwent a transplant for MF. A decline in QoL in the first 30 days was observed, with modest improvement at day 100. Few surveys have been completed at 1 year to date in this ongoing study. Collection of surveys past one year may be more informative regarding long-term impact of transplant on quality of life.
Mesa:Gilead: Research Funding; Novartis: Consultancy; Ariad: Consultancy; CTI Biopharma: Research Funding; Galena: Consultancy; Celgene: Research Funding; Promedior: Research Funding; Incyte: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal