Abstract
Parainfluenza viruses (PIV) ARE associated with severe respiratory tract infections among immunocompromised patients. However, the most perplexing feature of PIV following hematopoietic cell transplantation (HCT) is the dichotomous nature of risk for life-threatening infection. Specifically, the vast majority of patients will not require treatment of any kind whereas a small subset appears to be at such high risk of death that it has been questioned if successful treatment is even possible.
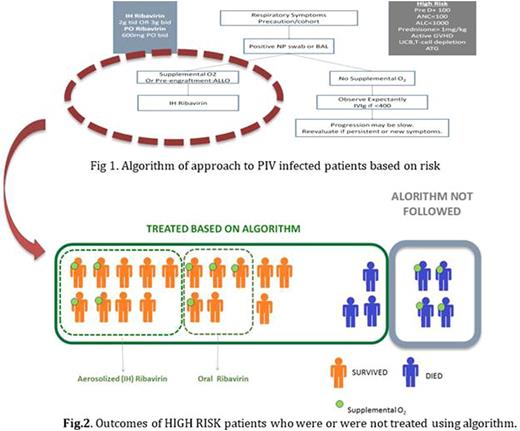
In 2007, we implemented a standardized approach to patients diagnosed with Parainfluenza virus (PIV) following HCT at Stanford University Medical Center (Fig 1) based on the need for supplemental oxygen and/or specific risk factors. We performed both a prospective analysis of cases of lower respiratory tract infection and a retrospective review of all 2495 patients transplanted during this period. The epidemiology, risk factors, clinical status, and outcome of the 115 PIV patients using this protocol from September 2007 through May 2015 shows that prudent, risk-stratified application of antiviral therapies in can result higher survival rates among patients with PIV post-HCT. Moreover, high risk patients who received aerosolized ribavirin had a lower mortality rate than those who were either not treated or received oral ribavirin.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal