Abstract
Background: Telmisartan (TMS) is an angiotensin receptor blocker (ARB), approved in 1998 for treatment of hypertension. Additional mechanisms of action were described post-approval, including PPARɣ agonism (by physical binding) and Rho Kinase inhibition (indirectly, at the level of gene expression). A third function is suppression of NADPH oxidase, causing increased Nrf2, a master regulator of anti-oxidant genes like superoxide dismutase. TMS's effect on blood pressure plateaus at 80 - 160 mg/day, and is minimal in normotensive subjects. Elimination of intact bioactive drug is via intestine and biliary tract, without liver enzyme involvement.
Importantly, TMS is anti-inflammatory and Treg inducing in various pre-clinical disease models, but has not been associated with immunosuppression or increased cancer risk during two decades of use. Given its excellent safety profile, described mechanisms of action, intact gut elimination, and pre-clinical effects, we reasoned that treatment prior to and during the acute post-transplant period could prevent severe acute graft-vs.-host-disease (GvHD) targeting the bowel, in recipients of related or unrelated donor (RD or URD) allogeneic hematopoietic stem cell transplants (allo-HSCTs) matched for 7/8 or 8/8 HLA.
Methods: An open label Phase I/II trial is designed to enroll 60 adult allo-HSCT patients for treatment with TMS 160 mg daily, starting 2 days pre-transplant, and continuing for 99 consecutive days. Subjects receiving > 28 consecutive days of TMS are considered evaluable. Patients can receive myeloablative or reduced-intensity regimens, and mobilized PBPC or marrow. Post-HSCT immunosuppression is tacrolimus and methotrexate. GvHD is evaluated at least weekly using standard grading / staging. Safety stopping criteria are non-relapse mortality (NRM), relapse or progression, failure to engraft, and drug intolerance (excluding reversible hypotension) within the first 180 days post-HSCT. The study is historically controlled vs. recent 3 year data from the John Theurer Cancer Center, and powered to detect 50% and 20% reductions in, respectively, Grade 3-4 (26%, historic) and Grade 2 (74%, historic) GvHD, as primary effect endpoints.
Exploratory endpoints include: Endotoxin Activity Assay (EAA, Spectral) for LPS in blood, soluble ST2 (a marker of inflammation previously associated with acute GvHD, PreSage EIA, Critical Diagnostics).
Early Results: To date, 12 patients (10 male and 2 female, ages 24 - 70) have been enrolled (Table 1). Ten have reached the 100 day post-transplant point. Six of 12 patients discontinued drug - 5 for non-medical reasons, prior to day 100 ("too many" pills and/or samples, forgot to take, did not feel good) - three of them after > 28 days of treatment. One other patient required dose reduction to 80 mg/d. Among all 12 enrollees, including those with < 28 days of TMS, there was one MDS progression to AML and one persistence of MDS. There was no graft failure or NRM.
Among 8 evaluable patients (i.e., > day 100 post-HSCT, > 28 days' TMS), there have been no cases of Grade 4 GvHD. Two patients receiving > 28 days of treatment experienced Grade 3 GvHD. One patient had only skin rash involvement; a second (Pt #3) had, at separated time points, rash, diarrhea, and elevated LFTs, with rapid response to steroid therapy. No patients developed Grade 2 GvHD during the 100 day acute period, although three patients developed chronic Grade 2 rashes after 100 days.
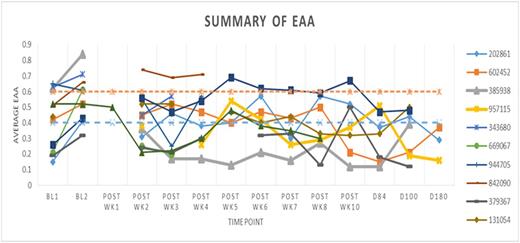
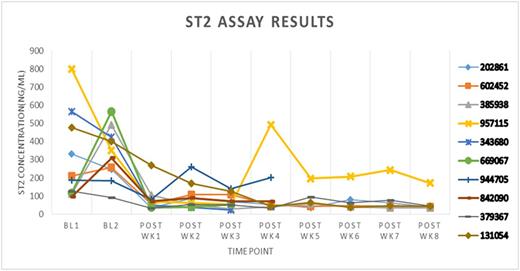
Exploratory endpoints revealed an increase in LPS (Fig. 1) and sST2 (Fig. 2) during the pre-transplant preparative regimen, reaching septic levels in some cases, but generally returning to normal or sub-septic levels during the post-transplant phase.
Discussion: Early results suggest safety of TMS, and possible reduced frequency and severity of GvHD with 28 - 100 days of treatment. The study continues, with close attention to day 180, 1 year, and longer term outcomes, in relation to blood markers and lymphocyte subsets. Retention of out-patient subjects is challenging due to complexity of post-HSCT regimens and symptoms, which may be overwhelming for some patients. Also, the absence of GvHD symptoms, possibly due to early acute phase TMS treatment, may reduce the incentive to continue TMS in some cases.
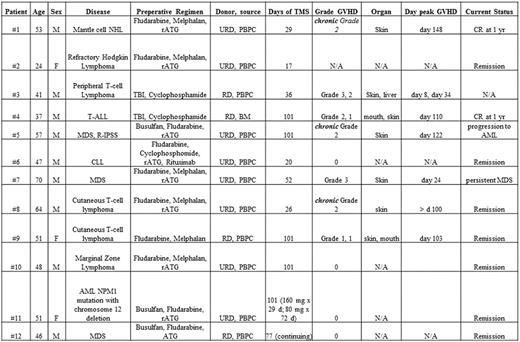
Characteristics of Study Patients
EAA (requires adequate leukocyte counts, typically unavailable at wk 1 post-HSCT)
EAA (requires adequate leukocyte counts, typically unavailable at wk 1 post-HSCT)
Schwartz:Hackensack University Medical Center: Patents & Royalties: provisional patent for use of telmisartan to prevent or treat GvHD. Iyengar:Hackensack University Medical Center: Patents & Royalties: I am on a provisional patent for the use of Telmisartan to treat Graft v. Host Disease.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal