Abstract
The use of PTCY is associated with reduced risk for GVHD in T cell replete NMA haplo-human stem cell transplants (HSCT); however, this intervention is still not sufficiently safe to justify treatment of non-malignant diseases or as a platform for organ transplantation. Furthermore, use of immune suppression to ameliorate GVHD likely adversely impacts anti-tumor immunity. Conversely, risk of rejection after NMA conditioning presents a major challenge for graft survival in T cell depleted (TCD) haplo-HSCT.
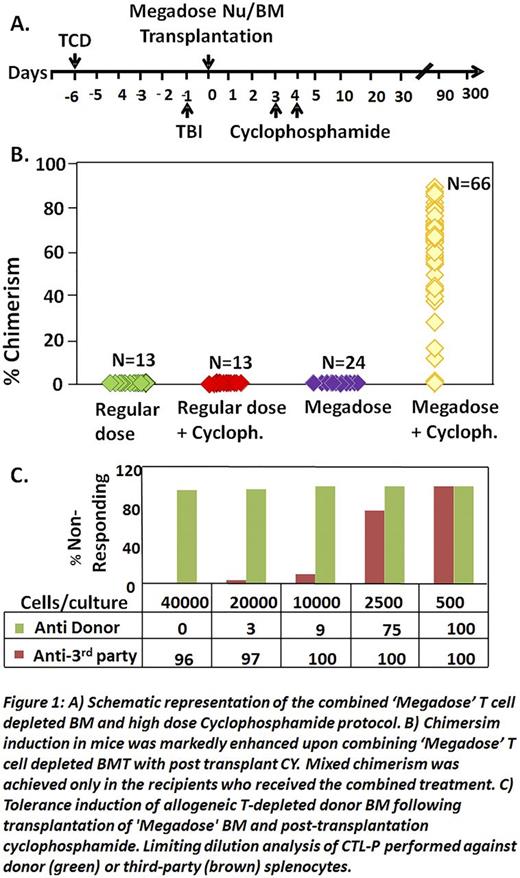
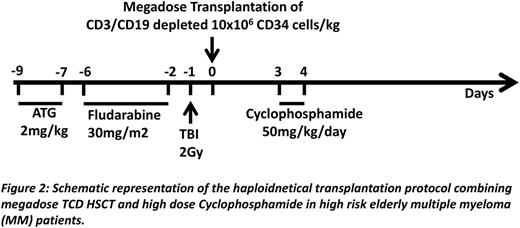
We now show in a total of 66 mice (9 experiments) that combining the power of megadose TCD HSCT with high dose PTCY (Fig.1A), enables marked and durable chimerism following NMA conditioning, while each modality alone was ineffective (Fig1B). Chimerism included all myeloid and lymphoid lineages, and LDA analysis of alloreactive T cells revealed specific immune tolerance towards donor stimulators (Fig.1C), also associated with acceptance of donor but not 3rd party skin. A similar protocol (Fig.2) was therefore developed for clinical use in patients with high risk hematologic malignancies.
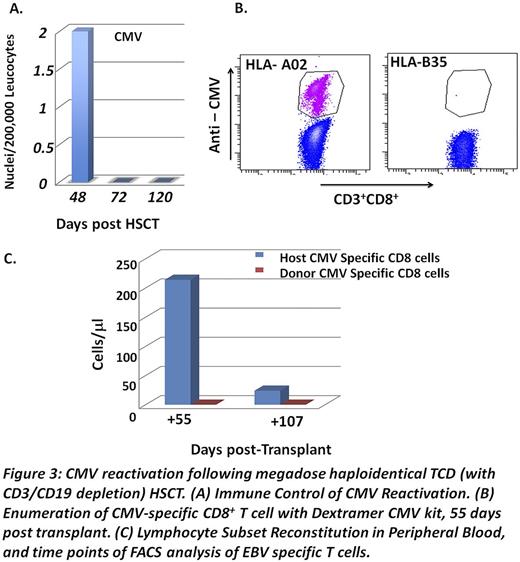
The first patient, a 54 yr old male with high-risk multiple myeloma (unfavorable cytogenetics, micromolecular disease and compromised renal function at diagnosis) was treated with 4 courses of Velcade-Thalidomide-Dexametasone and autologous HSCT, attaining complete remission with good performance status and normal renal function. Six months later, he received megadose (15.4 x106 CD34+ cells/kg) CD3/CD19 depleted (1.17 x105 CD3+T cells/kg) haloidentical PBPCs. Hematopoietic engraftment was achieved at day +15 with over 96% donor type chimerism during the first 6 months in the myeloid and B cell lineages. T cells during this period were predominantly of host type (10-23% donor type), gradually increasing to 63-72% at 9-12 months post transplant. EliSpot analysis of anti-donor CTLp revealed specific non-responsiveness. The patient overcame CMV (Fig.3A) and subsequently EBV reactivation without any treatment. Dextramer FACS analysis revealed that CMV specific CD8 T cells were exclusively of host origin (Fig3B-C). At 12 months, complete hematological remission and normal Free Light Chain ratio were confirmed.
The second patient, a 50 yr old male with MM achieved a partial remission after 4 courses of Lenalidomide-Dexametasone. He subsequently underwent autologous HSCT, and was put on Lenalidomide maintenance until relapse after 3 yrs. Following 8 courses of Bortezomib-Dexametasone he achieved CR. The patient received a megadose (10.8 x106 CD34+ cells/kg) of CD3/CD19 depleted (1.2x105 CD3+T cells/kg) haploidentcal PBPCs. Despite transient engraftment (50% donor cell on day +17), graft failure with autologous recovery (0.04% donor-type chimerism) was documented on day +30. This may be due to the extended treatment (38 months) with lenalidomide, but rejection cannot be excluded. After 5 months, this patient tolerated a second haploidentical HSCT (different donor) after standard myeloablative conditioning (ATG, treosulfan, thiotepa and fludarabine) and alfa/beta TCR/CD19-depleted PBPCs. Two months since the second HSCT, he shows no sign of GvHD, very good immunological reconstitution, excellent quality of life, and remains in complete remission. Collectively, our murine proof of concept data supported by clinical experience in the first high risk MM patient, suggests that combining megadose TCD HSCT with PTCY can enable prompt and durable engraftment with almost complete chimerism in the myeloid and B cell lineages. The marked level of host T cells persisting during the first 6 months after HSCT can provide anti-viral immune protection until thymus-derived donor T cells are generated. Furthermore, our ability to avoid post transplant immune suppression with this GVHD-free protocol, thereby attaining robust anti-viral immunity, could also potentially offer enhanced GVL effect, valuable for high risk MM patients.
The rejection experienced by the 2nd patient, although corrected by a 2nd myeloablative TCD HSCT, indicates that the conditioning must be fine-tuned to optimize engraftment in every patient.We are therefore testing, increasing TBI from 2Gy to 3Gy. Further studies will determine the efficacy of this approach in elderly MM patients, in non-malignant hematopoietic diseases, or as a prelude for organ transplantation and cell therapy.
*F.A and E.B.L contributed equally
BacharLustig:Yeda LTD: Patents & Royalties. Or Geva:Yeda LTD: Patents & Royalties. Reisner:Cell Source LTD: Consultancy, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal