Abstract
Extramedullary myeloma (EMM) is defined by the presence of plasma cells outside the bone marrow in a patient with multiple myeloma (MM) (Touzeau & Moreau, 2016). EMM is by itself a rare entity with cases involving central nervous system (CNS) being rarer, it has an estimated incidence of 1-1.8% of all MM cases (Majd et al, 2015). Its presentation in comparison to classical MM has an adverse prognosis including a shorter progression free survival (PFS) (Gozzetti et al, 2015) and overall survival (OS) with a mean life expectancy of 1.5-6 months (Majd et al, 2015). Currently there are not international treatment guidelines for this disease.
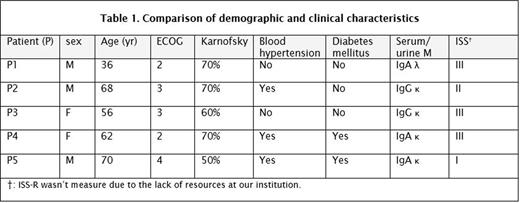
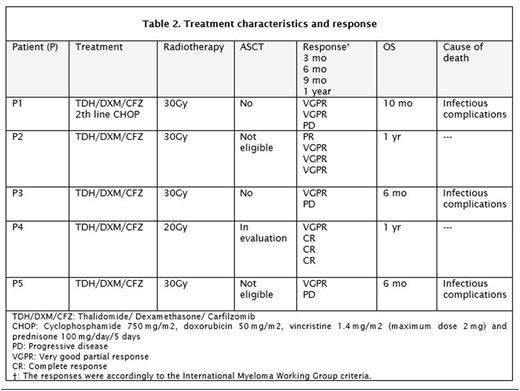
The aim of this report is to illustrate our experience at a single institution in Mexico, presenting five cases of CNS-EMM treated with proteasome inhibitor (carfilzomib) in combination with thalidomide, dexamethasone plus radiotherapy. The patient's characteristics and the treatment regimen are shown in table 1 and 2. Within three months, following the criteria to the international multiple myeloma working group (IMMWG), all five patients accomplished a positive response (one partial response [PR] four very good partial responses [VGPR]), at sixth months two patients improved their response from previously PR to a VGPR and from VGPR to complete response (CR), one sustained its VGPR and after this period of time two patients had progressive disease (PD) and died due to infectious complications. After the tenth month a third patient also died of infectious complications. By the end of this report two patients still alive, one is being evaluated for autologous stem cell transplantation (ASCT) and the other one wasn't eligible according to European Group for Blood and Marrow Transplantation (EBMT) risk score and will continue treatment without ASCT.
Previously reported cases in the literature with old chemotherapy regimens shown to have median survival of 1.5 months (Petersen et al 1999), nowadays the recommended therapy is based on triplet induction therapy followed by high-dose melphalan/ASCT, a triplet consolidation therapy and maintenance treatment with lenalidomide (Touzeau & Moreau, 2016); so far there is no evidence of proteasome inhibitors penetrating the blood brain barrier (Nooka et al, 2013) and this might be the reason why the literature strongly recommends the use of lenalidomide as this one does penetrate into the CSF after oral administration (Muscal et al, 2012), we did not use lenalidomide due to the high monetary cost of the treatment but instead we use thalidomide in combination with carfilzomib and dexamethasone with improved OS in three of our five patients (VGPR + CR).
Despite the small number of patients presented in this report and the lack of cytogenetics or FISH information, we think this might be a good therapeutic approached in patients with CNS-EMM, especially in those scenarios with not accessible resources or absence of clinical trials. Further studies evaluating the addition of monoclonal antibodies (daratumumab, elotuzumab) need to be done in order to improve the OS and PFS in this group of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal