Abstract
Introduction:
Bortezomib (Bor) is widely used as a frontline therapy and improves outcome in treatment of multiple myeloma (MM). Although Bor was initially approved for intravenous (IV) administration, subcutaneous (SC) administration is currently standard practice, based on the randomized trial showing that SC exhibited similar overall response rate (ORR) and lower incidence of peripheral neuropathy (PN) compared with IV among relapsed MM. On the other hand, the other groups revealed that IV achieved better response than SC after 3 cycles of therapy, and overcame SC resistance in some patients. In this study, to clarify whether SC actually improves the outcome of MM patients, we compared the efficacy and side-effects between IV and SC Bor administration.
Patients and methods:
We retrospectively analyzed 46 MM patients including newly-diagnosed (n=37) and relapsed (n=9) cases treated with at least two cycles of Bor-containing regimens at The University of Tokyo Hospital from August 2007 until June 2016. Bor-containing regimens consisted of Bor only (Bor 1.3 mg/m2, days 1, 4, 8, and 11, repeated every 21 days), Bor/dexamethasone (Dex) (BD, Bor 1.3 mg/m2, days 1, 4, 8, and 11; Dex 40 mg/body, days 1-4, 9-12, repeated every 21 days), Bor/cyclophosphamide (Cy)/Dex (CyBorD, Bor 1.3 mg/m2, Cy 300 mg/m2, Dex 40 mg/body, days 1, 8, 15, and 22, repeated every 35 days), Bor/lenalidomide (Len)/Dex (VRD, Bor 1.3 mg/m2 days 1, 4, 8, and 11; Len 25 mg/day days 1-14; Dex 40 mg/body, days 1, 8, and 15, repeated every 21 days), and Bor/melphalan (Mel)/prednisolone (PSL) (VMP, Bor 1.3 mg/m2, days 1, 8, 15, and 22, Mel 6 mg/m2, PSL 40 mg/m2, days 1-4, repeated every 35 days). Response categories were in accordance with International Myeloma Working Group uniform response criteria. Bor responders were defined as those who achieved more than 5% of monoclonal protein reduction within the first two courses of treatment. To measure the initial response among Bor responders, we calculated per-cycle reduction rate of monoclonal protein until achieving maximal response. For statistics, t-test, Fisher exact test and Log-rank test were utilized.
Results:
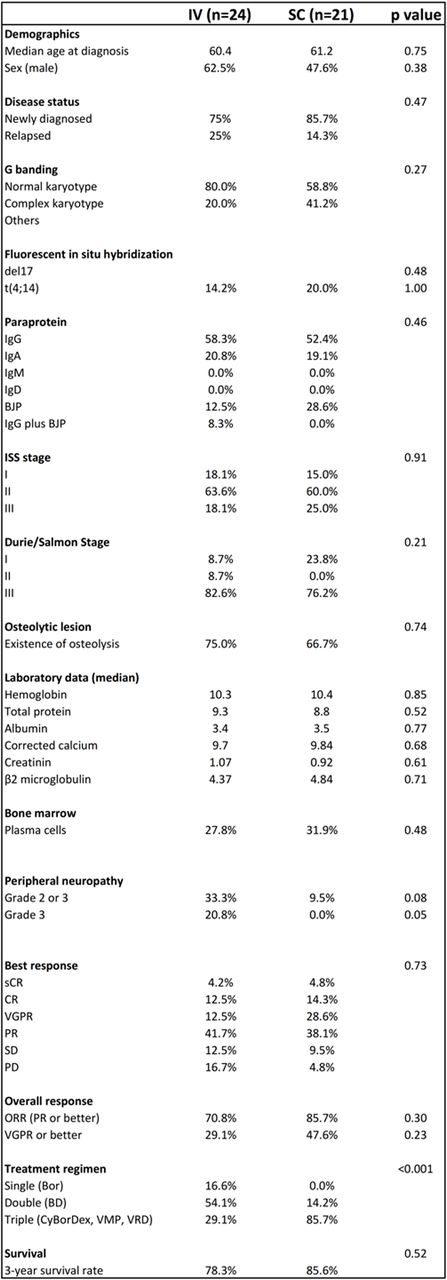
Of 46 patients, 24 (52.2%) and 21 (45.7%) patients received IV- and SC-Bor, respectively. One case in which administration route was switched from IV to SC due to vasculitis was excluded from further analysis. Thirteen patients (54.1%) in IV received BD, while 18 patients (85.7%) in SC received triple combination therapy (CyBorD, VRD, or VMP). Between IV and SC, there was no significant difference in age, laboratory data, disease status (newly diagnosed or relapsed) and clinical staging (ISS and Durie/Salmon) (Table 1). Grade 3 PN was more frequently observed in IV (n=5, 20.8%) than in SC (n=0, 0%) (p=0.05). ORR (partial response (PR) or better) was 70.8% in IV and 85.7% in SC (p=0.30). Very good PR (VGPR) or better response was achieved in 29.1% and 47.6% for IV and SC, respectively (p=0.23). Three-year survival rate was 78.3% in IV and 85.6% in SC (p=0.52). Among Bor responders (n=40), no significant difference was observed between IV and SC in per-cycle monoclonal protein reduction rate (25.4% vs 36.8%, p=0.08), median number of courses (3.1 vs 2.8, p=0.50) and median number of Bor administration (11.2 vs 10.7, p=0.75) until achieving maximal response. Subgroup analysis among newly-diagnosed MM patients treated with IV-BD (n=10) and SC-triple (n=16; 9 with CyBorD, 5 with VMP, 2 with CyBorD followed by VRD) showed that there was no significant difference in patient characteristics, ORR (70% for IV-BD and 87% for SC-triple (p=0.34)), and VGPR or better response rate (40% for IV-BD and 37% for SC-triple (p=1.0)). Three-year survival rate was 77.8% and 78.6% for IV-BD and SC-triple, respectively (p=0.70). Additionally, among Bor-responders, no significant difference was observed between IV-BD and SC-triple regarding per-cycle reduction rate of monoclonal protein (26.6% vs 31.0%, p=0.52), median number of courses (2.6 vs 2.8, p=0.59), and median number of Bor administration (9.6 vs 11.0, p=0.42) until achieving maximal response.
Conclusions:
In this retrospective study, IV-BD exhibits similar initial and maximal response compared with SC-triple among newly-diagnosed MM patients, while SC reduces the incidence of severe PN. Taking into account the therapeutic superiority of Bor-based triple drug regimen, our findings suggest the stronger therapeutic potency of IV-Bor administration.
Nakamura:Janssen Pharmaceutical K.K.: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal