Abstract
Introduction: Neutrophil extracellular traps (NETs) are structures composed of DNA, histones, and bactericidal factors that are expelled by neutrophils in order to trap and neutralize bacteria. NETs play a role in host defense by trapping and killing infecting bacteria and inactivating bacterial virulence factors. Activation of the coagulation cascade by these components can lead to "immunothrombosis" and facilitate the containment and destruction of bacteria within a fibrin clot. Although extracellular nucleosomes (structures consisting of DNA wound around a histone protein core) within NETs can contribute to host defense, they can also play a role in disease pathology by leading to inflammation, endothelial damage, and pathological thrombosis. Disseminated intravascular coagulation (DIC) is a condition characterized by systemic activation of the coagulation and fibrinolytic systems that can occur in conjunction with several underlying conditions, including sepsis. Links between infection, host response, and systemic coagulation, extracellular nucleosomes may play a significant role in the pathophysiology of sepsis-associated DIC. The purpose of this study was to quantify extracellular nucleosomes in the plasma of patients with sepsis-associated DIC.
Materials and Methods: Citrated, de-identified plasma samples were collected from patients with sepsis and suspected DIC at ICU admission and on ICU days 4 and 8 under an IRB approved protocol. DIC score was evaluated in each sample using the ISTH scoring algorithm incorporating platelet count, PT/INR, fibrinogen (Recombiplastin, Instrumentation Laboratory, Bedford, MA), and D-Dimer (HyphenBioMed,Neuville-Sur-Oise, France). Plasma from healthy individuals was purchased from a commercial laboratory (George King Biomedical, Overland,KS). Nucleosomes in plasma were measured using the Cell Death Detection ELISA (Roche Diagnostics, Indianapolis, IN). The correlation of variation for both intra-assay and inter-assay variation was <15%.
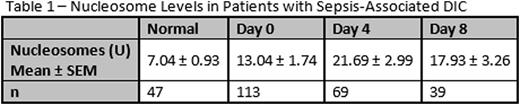
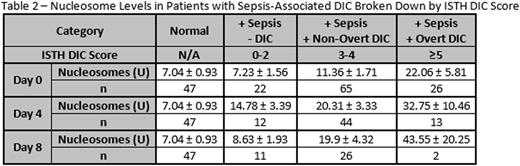
Results: Nucleosomes were significantly elevated in patients with sepsis and suspected DIC compared to healthy individuals on ICU days 0 (p = 0.028), 4 (p < 0.0001), and 8 (p = 0.013). Results are shown in Table 1. When patients were categorized according to ISTH DIC score, a non-significant trend towards increasing nucleosomes with increasing DIC score was observed. Nucleosomes were significantly elevated in patients with overt DIC compared to normal individuals on ICU day 0 (p = 0.02). On ICU day 4, nucleosomes were significantly elevated in patients with both overt and non-overt DIC compared to healthy individuals (p < 0.01). Results are shown in Table 2. Furthermore, nucleosome levels correlated significantly (r >0.2, p<0.05) with factors involved in inflammation and coagulation. Nucleosomes correlated significantly with D-Dimer, prothrombin fragment F1.2, IL-8, and IL-10. No significant correlation was observed between nucleosomes and IL-2, IL-4, IL-6, VEGF,IFNγ, TNFα, IL-1α, IL-1β, MCP1, and EGF.
Conclusion: Plasma nucleosome levels were elevated in patients hospitalized with sepsis and suspected DIC, and a trend towards increasing circulating nucleosome levels with increasing DIC score was observed. This supports the hypothesis that nucleosomes contribute to the pathophysiology of sepsis-associated DIC. The correlation of nucleosomes with the infection markers and a subset of inflammatory markers suggests that the presence of nucleosomes in the plasma of patients with sepsis-associated DIC may be due to specific, infection-related processes and not to general inflammatory processes. Additionally, the correlation of circulating nucleosome levels with markers of thrombin generation and fibrinolysis suggests that nucleosomes may play a role in the activation of coagulation observed in patients with sepsis-associated DIC.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal