Abstract
Introduction
Due to immune dysregulation and subsequent increased risk of infection associated with chronic lymphocytic leukemia (CLL), both the CDC and ACIP recommend patients with CLL receive the 13- valent pneumococcal conjugate vaccination (PCV13). The effect of ibrutinib, a selective inhibitor ofBruton'styrosine kinase (BTK) which effects B-antigen receptor (BCR) signaling, on IgG antibody responses to pneumococcal vaccination in patients with CLL has not been evaluated. The aim of this study was to evaluate the immune response to PCV13 in patients receiving in ibrutinib and CLL control patients (no active treatment).
Methods
Study patients were enrolled on an IRB approved protocol at Georgia Cancer Center. Specific inclusion criteria a) histological confirmed CLL as defined by WHO classification b) no current therapy (CLL control) c) ibrutinib 420 mg/day (active) d) ECOG ≤ 2. Exclusion criteria a) Previous vaccination with PCV13 within 2 years b) anti-CD20 therapy within last 6 months c) IVIG therapy within 6 weeks. Serum pneumococcal antibody assessment was performed by microsphere photometry on subjects at Day 0 and Day 30 days following vaccination. Antibody specific serotypes (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23) were determined by ELISA. Pre- and post- vaccination expression of BTK and HACS1 (hematopoietic adapter containing SH3 and SAM protein) of CD19+ lymphocytes was determined by Western Blot analysis. The primary objective was to determine whether concurrent administration of PCV13 in patients receiving ibrutinib generates a ≥ 2 fold increase in ≥ 3 of pneumococcal serotypes as compared to the control group. Secondary objectives were to evaluate expression BTK and HACS in both study groups.
Results
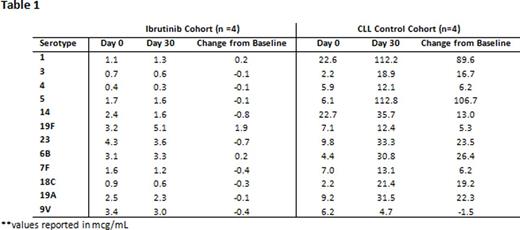
At interim analysis, 9 patients have been enrolled to date, with 8 patients (n=4 ibrutinib, n=4 CLL control) having completed pre and post-vaccination assessments. The median patient age was 69 yo(range: 53-77 yo) with 75% ≥ 65 yo. All CLL control patients (4/4) generated a ≥ 2 fold increase in ≥ 3 of pneumococcal serotypes, whereas (0/4) of ibrutinib patients generated an adequate immune response to PCV13 (p=0.029; Fisher exact). See Table 1 for mean serotype changes in both patient cohorts.
Conclusions
Preliminary data suggests that patients who are receiving ibrutinib group do not generate an effective immune response to PCV13 vaccination compared to untreated CLL controls. This may have an impact on susceptibility and response to infection with pneumococcal infection as well.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal