Abstract
Introduction: Bendamustine hydrochloride injection (Treanda®) was approved in 2008 by the US Food and Drug Administration (FDA) for the treatment of patients with chronic lymphocytic leukemia (CLL) and relapsed/refractory indolent B-cell non-Hodgkin's lymphoma (NHL). Postmarketing pharmacovigilance (PV) is required for products approved by the FDA to meet regulatory and contractual requirements. Here we report on the real-world safety profile of bendamustine to better understand its long-term safety. PV data spanning a 9-year period were reviewed, with an emphasis on safety signals that led to product label revisions since initial FDA approval.
Methods: A total of 12 quarterly postmarketing periodic adverse drug experience reports (PADERs) spanning March 20, 2008, to March 31, 2011, and three subsequent annual PADERs for the period ending March 31, 2015, were included. PV data were collected from foreign and domestic Teva-sponsored clinical studies, special drug-access programs, surveys, other systems in which patients are requested to provide adverse event (AE) information, spontaneous reports, and the scientific literature.The PV data collected were housed in the Teva Pharmaceuticals safety database. AEs were coded according to Medical Dictionary for Regulatory Activities terms and classified as serious or nonserious and expected or unexpected (ie, AEs not included in the product label at the time of the report or those that occurred at a higher incidence than previously reported).
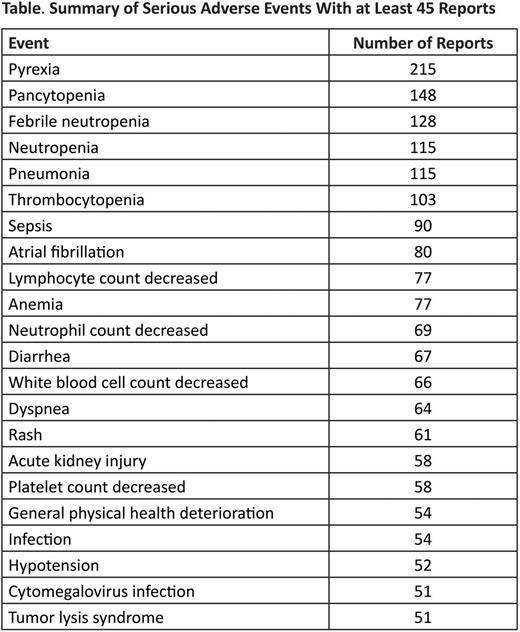
Results: As of January 6, 2015, 252,229 patients have been treated with bendamustine worldwide. Of the 3679 patients (1.5%) who experienced AEs over the entire postmarket monitoring period, 2441 individuals (1%) experienced serious and unexpected AEs. There were 364 cases classified as serious and expected, and 874 classified as nonserious. The most frequently reported serious AEs are summarized in the table. Serious AEs that resulted in label updates included Stevens-Johnson syndrome (n=33), toxic epidermal necrolysis (n=12), extravasation (n=7), and secondary neoplasm (n=272). A recent safety review was conducted to evaluate the potential relationship between bendamustine administration and drug reactions with eosinophilia and systemic symptoms (DRESS). Nine reports of DRESS have been received since 2008. No clear causal relationship could be established between bendamustine and the development of DRESS because of concomitant medications known to be associated with DRESS in all cases. Information about preventive measures for tumor lysis syndrome (TLS) was revised during the PV period; 51 instances of serious TLS were reported during the monitoring period.
Conclusions: This overview of AE reports collected from over 250,000 patients over 9 years suggests that the overall safety profile of bendamustine is consistent with known toxicities, with the majority of serious AEs associated with myelosuppression and infections. Although this review may be limited by voluntary reporting, the AEs reported for bendamustine in a large, heterogeneous population provide a much broader understanding of its safety profile. Based on this observational data, bendamustine appears to have a favorable risk-benefit profile and remains a useful option when considering a management strategy in patients with CLL and indolent B-cell NHL.
Sponsor: Teva Branded Pharmaceutical Products R&D
Martin:Acerta: Consultancy; Janssen: Consultancy, Honoraria, Other: travel, accommodations, expenses; Gilead: Consultancy, Other: travel, accommodations, expenses; Novartis: Consultancy; Celgene: Consultancy, Honoraria; Teva: Research Funding. Kahl:Infinity: Consultancy; Gilead: Consultancy; Juno: Consultancy; Pharmacyclics: Consultancy; Celgene: Consultancy; Seattle Genetics: Consultancy. Pathak:Teva Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal