Abstract
The clinical course of chronic lymphocytic leukemia (CLL) is highly heterogeneous, from stable to a rapidly progressive. The variety of prognostic factors has been already described, nevertheless they are not fully efficient in predicting the course of CLL, especially when the disease is diagnosed at an early stage. Recently, beside well established cytogenetic prognostic factors, novel molecular mutations of predictive value have been identified. Many of them have been thoroughly characterized, including TP53 mutation, which is commonly considered as a strong, negative prognostic factor. The use of next-generation sequencing technology has also revealed previously unknown genomic alterations, such as neurogenic locus notch homolog protein1 (NOTCH1), splicing factor 3B subunit 1 (SF3B1) or myeloid differentiation primary response 88 (MYD88). These new mutations could partly explain the CLL heterogeneity and help in identifying clinically relevant groups of patients.
The aim of the study was to characterize CLL patients with NOTCH1, MYD88 and SF3B1 mutations with regard to molecular and immunological prognostic markers in CLL. Peripheral blood mononuclear cells (PBMCs) were obtained from 369 CLL patients at the moment of diagnosis and the median age reached 65 years. Sixty percent of the patients were male. Distribution of disease stages according to the Rai classification was the following: 0 stage=93, I stage=52, II stage=69, III stage=14, IV stage=26. Clinically, the prognostic significance in terms of time to first treatment (TTFT) was assessed for 202 CLL patients.
DNA samples were extracted from PBMCs obtained after Ficoll density gradient centrifugation. NOTCH1 c.7544_7545delCT (n=316) in PEST domain (exon 34) and MYD88 L265P (n=323) mutations were investigated by ARMS PCR. Screening for SF3B1 (n=364) mutations K700, E622/R625 and H662/K666 (exons 14 and 15) were performed using HRM analysis and the results were confirmed by Sanger sequencing. The IGHV gene mutations were investigated by Sanger sequencing.
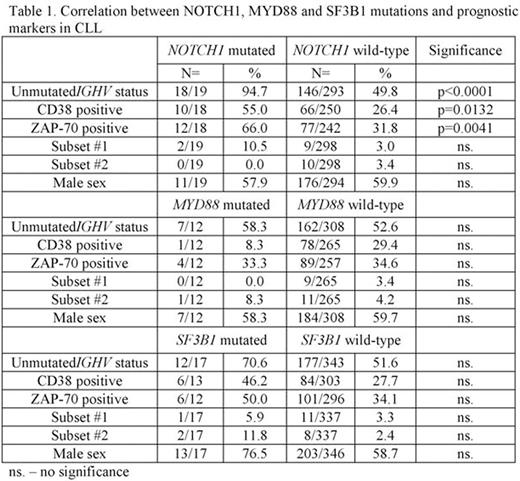
NOTCH1 mutations were found in 19/316 (6.0%) patients. Patients harbouring NOTCH1 mutations prevalently have unfavourable prognostic factors including unmutated IGHV gene status, expression of CD38 (>30%) and expression of ZAP-70 (>20%). The complete analysis of correlations between NOTCH1, MYD88 and SF3B1 mutations and prognostic markers in CLL are presented in Table 1. Analysis of IGHV subsets in patients with NOTCH1 mutation revealed frequent presence of subset #1 in n=2/19 (10.5%), which is associated with particularly poor prognosis in CLL. Patients belonging to subsets #5, #6, #201 and #202 were also present, each in single NOTCH1 mutated CLL case (5.2%). MYD88 mutation occurred in 12/323 (3.7%), of whom one patient was characterized as subset #2 and another as subset #4. MYD88 mutations were nearly equally distributed in patients with mutated/unmutated IGVH status (5 vs. 7). SF3B1 mutations occurred in 17/364 (4.7%) patients, furthermore two of them carrying negative prognostic features of subsets #2 and one subset #1. Patients belonging to subsets #3 and #6 were also present.
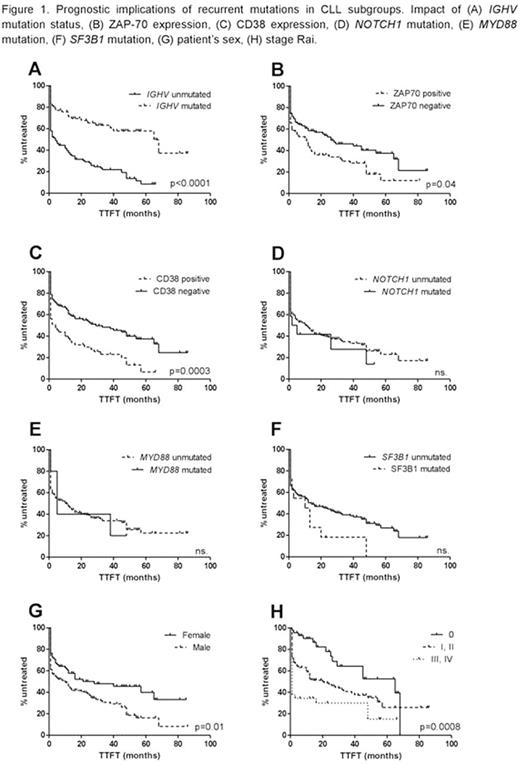
Certain negative prognostic factors accompany poor clinical outcome. The assessment of median TTFT revealed the significant differences between patients from various prognostic groups (Fig. 1). Patients with unmutated IGHV gene status were characterized by significantly shorter TTFT than patients harbouring the mutation (p<0.0001). The similar correlation occurs in patients with ZAP-70 positive (p=0.04) and CD38 positive (p=0.0003). There were no significant differences in patients with mutated and unmutated NOTCH1 and MYD88, while in patients harbouring SF3B1 mutation the tendency to lower median TFTT was revealed (p=0.08). Interestingly, the significant difference in median TTFT was observed in groups of men and women, showing the better outcome in female patients (median 10 vs 28 months, p=0.01).
NOTCH1 and SF3B1 mutations accompany certain biological markers of unfavourable prognosis. Undeniably, the mutations may contribute to the identification of poor-risk CLL patients and in combination with conventional lesions of CLL may be the key to accurate estimation of the disease prognosis.
Acknowledgments: This work was supported by Polish Ministry of Science and Higher Education Scientific Grant "The best from the best" ("Najlepsi z Najlepszych") No. 506-0000-39-0000.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal