Abstract
The AZA-001 study showed that azacitidine has a significant role in prolonging overall survival (OS) in High-Risk Myelodysplastic Syndromes (HR-MDS), particularly in patients with unfavorable cytogenetics, even in the subset of patients without any recorded response except progression. Otherwise, the benefit of azacitidine treatment in OS of HRMDS shows a lack of improvement in survival over the years in most of the patients in a Multicentric Spanish Study Group.
Since in our institution we had 34 patients who received Azacitidine for HR-MDS and 20-30% blast Acute Myelogenous Leukemia (AML) during last 36 months, we performed a retrospective analysis of our current long-term treatment patients currently taking Azacitidine, to understand their characteristics. We used WHO classification, IPSS- R score, bone marrow (aspirate) blast count, conventional cytogenetics-based risk, performance status (ECOG) and transfusional dependence.
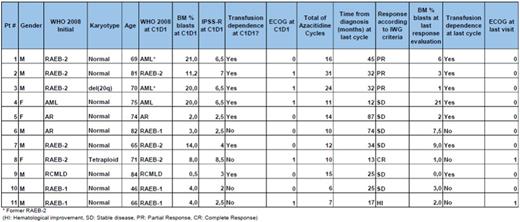
We studied 11 patients with MDS who received at least 06 cycles of Azacitidine,(75 mg/ m2 D1 to D7) until data cut on 1st August 2016. The median age was 71 (range 46-84 years) and 72 % of the patients are male. WHO 2008 classification before beginning of treatment was: 3 AML (2 former RAEB-2 with recent progression), 03 Refractory Anemia with Excess Blasts-2 (RAEB-2), 02 Refractory Anemia with Excess Blasts-1 (RAEB-1), one Refractory Cytopenia with Multilineage Dysplasia (RCMLD) and 02 Refractory Anemia (RA). All but one patient had "good" cytogenetic risk and IPSS-R ranged from 2 to 8,5 (low to high risk), median 4,7 points.
Regarding treatment, patients received from 6 to 31 cycles of Azacitidine, with responses according to IWG including one complete response (CR), 03 partial responses (PR), 06 stable diseases (SD) and one Hematological improvement (HI). Transfusion dependence was present at the beginning of treatment in 07 cases, and no change in this parameter happened until those patient's last visit. Performance Status was very good (ECOG=0) in 06 patients before the first dose of azacitidine and good (ECOG-1) in the remaining subjects. At data cut, we had 09 patients with ECOG=0 in the group.
This analysis showed that patients had no real improvement of disease parameters since 07 of 11 did not achieve even a partial response, and the same number of patients stay as transfusion-dependents. However, even the worse IPSS-R classified patients had at least a better quality of life and they survived at least one year with treatment. The main finding of this evaluation is that patients who achieve a response other than progression or refractoriness have a good cytogenetic risk, most of them with normal Karyotype, confirming literature.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal