Abstract
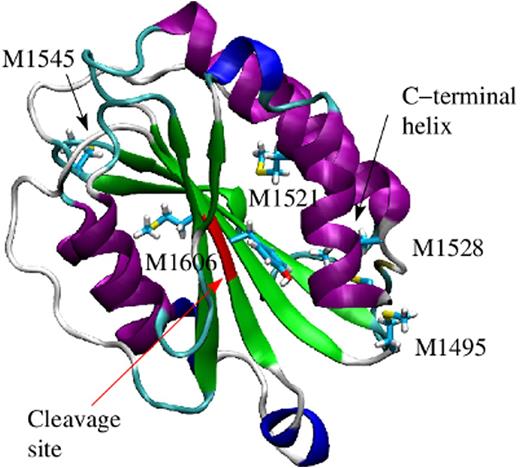
Inflammation is a ubiquitous phenomenon, resulting from such common disease states as obesity, injury, and sepsis, and it is associated with an elevated risk of thrombosis. During inflammation, neutrophils are activated and produce oxidants, such as hydrogen superoxide, which is then converted to hypochlorous acid (HOCl) through the action of the enzyme myeloperoxidase. This leads to modifications in the structure of coagulation factors, which is mainly due to the conversion of methionine residues to methionine sulfoxide. Recent experimental evidence indicated that oxidative conditions increase the ability of von Willebrand Factor (VWF) to tether platelets. This was linked to the oxidation of methionine residues in the A1, A2 and A2 domains of VWF (Fu et al. Blood 2011). These domains are key in regulating the platelet-tethering function of VWF. The A3 domain attaches to the exposed collagen at the site of injury, while the A1 domain binds to the platelet surface receptor GpIbα. On the other hand, the A2 domain, sandwiched between the two, contains a proteolytic target site for the metalloprotease ADAMTS13 (Figure 1), which cleaves it once the A2 domain is unfolded under tensile force. The molecular mechanism behind the oxidation-induced activation of VWF is currently not understood. In particular, it is not clear whether oxidation activates the A1 domain itself or whether oxidation removes the inhibitory function of neighboring domains.
In this work, we used an assay based on a parallel plate flow chamber to explore which inhibitory mechanisms of A1 are removed by oxidation. Then, we used molecular dynamics simulations and free energy perturbation methods to propose a model how oxidation affects the structure of the A2 domain favoring activation of VWF. Experimental evidence points out that neighboring domains of A1, in particular the A2 and A3 domains, inhibit its platelet-binding function (Auton et al. J. Biol. Chem. 2010). For this reason, constructs of VWF containing the A1 domain with or without the neighboring A2 or A3 domains were expressed in Chinese hamster ovary cells and anchored on a surface. Oxidation was induced by incubating the surface with HOCl. Beads coated with GpIbα were then washed over the surface at different shear rates while their trajectories were monitored. The results indicated that oxidation has little effect on the rolling behavior of beads when washed over the isolated A1 domain. However, oxidation caused beads to roll slower and accumulate more on surfaces coated with constructs where the A1 domain was flanked by the A2 and A3 domains.
The molecular mechanism how oxidation removes the inhibitory function of the A2 domain was explored through molecular dynamics simulations. The A2 domain presents a globular fold consisting of a central β sheet surrounded by α helices (Figure 1). Oxidation of methionine residues causes the C-terminal helix to separate from the rest of the protein at a lower tensile force. Furthermore, free energy perturbation calculations indicated that the A2 domain is more likely to be found in the denatured state when its methionine residues are oxidized. Although under normal conditions the A2 domain is cleaved by ADAMTS13 when unfolded, oxidizing agents increase its resistance towards proteolysis due to the oxidation of a methionine residue in the cleavage site (Chen et al. Blood 2010). Taken together, oxidation destabilizes the A2 domain, which causes its separation from the A1 domain, thus removing its inhibitory function. Understanding the molecular basis of how oxidation increases the pro-thrombotic activity of VWF could provide a target for future therapeutics aimed specifically at mitigating the thrombotic complications seen in many inflammatory conditions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal