Abstract
Introduction:
Interferon (IFN-α) is frequently used in various hematologic neoplastic disorders either at failure of first-line treatment or as first-line therapy in selected patients. IFN-α has shown significant activity in treatment, but the therapy is handicapped by high dropout rates due to side effects and cumbersome dosing schedules. Pegylated (peg) IFN- α is formulated by the covalent binding of one 40 kDa branched PEG-polymer to a lysine side chain of IFN- α. This modification prolongs serum half-life and patient exposure to medicine, decreases renal excretion, thus allowing for weekly administration, acceptable toxicity, tolerability and activity profiles.
The results of peg-IFN in polycythemia vera (PV) and essential thrombocythemia (ET) in phase 2 studies are encouraging, with molecular responses in some patients. There is at least one phase 3 study of hydroxyurea versus peg-IFN as first-line cytoreductive therapy for PV. In spite of ongoing international efforts to evaluate peg-IFN in PV, there is limited experience on peg-IFN use in other hematologic neoplasms.
Method: <>All patients who were put on peg-IFN in our center were found in our institutional electronic database. The patients had been recorded prospectively. All patients who had been treated with peg-IFN had been approved by national health authority and all patients had given informed written consent. Responses were evaluated by ELN (chronic myeloid leukemia), IWG-MRT (PV and ET), EUMNET (myelofibrosis), Nordic proposal criteria (hypereosinophilic syndrome), modified consensus criteria (systemic mastocytosis) (Am. J. Hematol 2009;84:790), Cheson criteria (lymphomatoid granulomatosis) and PET response criteria (Erdheim Chester disease) standardized criteria. Adverse effects were classified according to Common Terminology Criteria for Adverse Events (CTCAE4.0). Categorical and continuous data were expressed as ratio (%) and median (range). Statistical analyses were done by SPSS v17 (Chicago, IL).
Results:
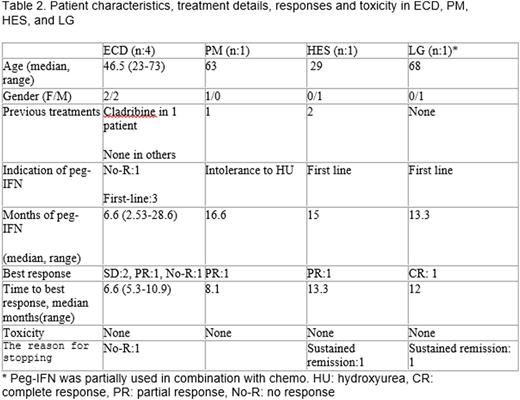
There were 30 patients with 8 different hematologic neoplasms: 12 PV, 6 ET, 2 chronic myeloid leukemia (CML), 3 systemic mastocytosis (SM), 4 Erdheim-Chester disease (ECD), 1 primary myelofibrosis (PM), 1 hypereosinophilic syndrome (HES), and 1 lymphomatoid granulomatosis (LG). Detailed descriptive data, toxicity and treatment results are presented in table 1. Responses were observed in all disease categories. Toxicities were frequent in PV/Et, including significant autoimmune disorders (nephritic syndrome, autoimmune hepatitis)
Conclusion:
Peg-IFN is effective in many hematologic neoplastic disorders. Unfortunately, toxicity is a major concern in this treatment.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal