Abstract
BACKGROUND - AIM
Ruxolitinib can be effectively used for the treatment of splenomegaly or constitutional symptoms in patients with myelofibrosis. Its main side effect is myelosuppression which leads to dose modifications. Real-life data from the use of ruxolitinib in every-day practice were analysed to assess efficacy, safety, dose modifications or reasons for treatment discontinuation.
PATIENTS - METHODS
A retrospective observational study was conducted in patients with primary or secondary myelofibrosis, treated in 13 sites. Statistical analysis was done with non-parametric tests using SPSSv17 software. A total of 116 patients, diagnosed between 1993 and 2016 were included in the analysis. The male:female ratio was 2:1, median age at diagnosis was 63 years (range 27 to 82) and median duration of ruxolitinib therapy was 15.3 months. The study expands over 664.2 patient-years of observation, including 176.7 patient-years while on ruxolitinib.
RESULTS
The diagnosis, revisited according to the WHO 2008 criteria, was primary myelofibrosis in 56%, post-essential thrombocythemia myelofibrosis in 22% and post-polycythemia vera myelofibrosis in 22% of patients. At the beginning of ruxolitinib treatment, the international prognostic scoring system (IPSS) risk category was low (6.2%), intermediate-1 (26.5%), intermediate-2 (39.8%), high (23%). Constitutional symptoms were present in 48% of patients at diagnosis and in 73% of them at the onset of ruxolitinib therapy. Median spleen size was 5cm (centimeters) below costal margin at diagnosis and 12cm at onset of ruxolitinib.
Ruxolitinib starting dose was 5mg (16%), 10mg (18%), 15mg (31%), 20mg (35%) twice daily (BID), while the final dose was <5mg (5%), 10mg (25%), 15mg (23%), 20mg (23%) and 25mg BID (2%). Starting dose was modified in 77% of patients; the median time to first modification was 3.7 months, while 90% of the modifications had occurred within 14.8 months from onset of therapy.
Ruxolitinib was permanently discontinued in 29% of patients, due to progressive disease in 6.4%, transformation to acute leukemia in 2.7% and death in 8.2% of patients. Side effects (including anemia, thrombocytopenia, investigator decision, patient preference) was the reason for treatment discontinuation in 5.5% and lack of response in 2.7% of patients.
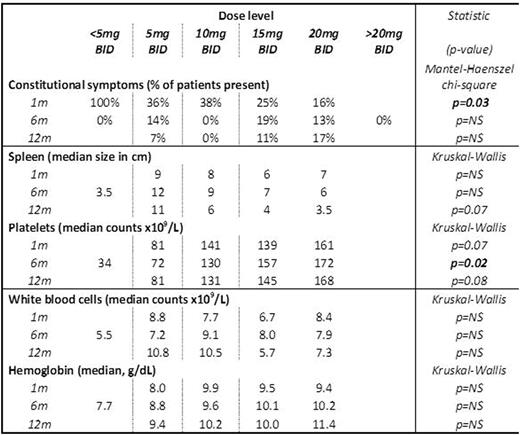
Efficacy (reduction in constitutional symptoms and spleen size) and hematological toxicity (values of platelets, white blood cell counts, hemoglobin) of ruxolitinib was assessed after 1, 6 and 12 months of treatment (table 1). Thrombocytopenia (PLT<130 x 109/L) was present in 26% of patients at the beginning of treatment, in 46% at 6th month and in 42% at the 12th month, while grade III and IV thrombocytopenia (PLT<50 x 109/L) was present in 4%, 11% and 5% respectively.
Constitutional symptoms after 1 month were significantly less frequent with increased doses of ruxolitinib, whereas there was no statistically significant difference among the various doses at 6 and 12 months of treatment. Platelet counts were significantly lower in patients taking 5mg BID at 6 months of treatment (p=0.02), and they also tended to be lower in 1 and 12 months (p=0.07, p=0.08 respectively). White blood cells and hemoglobin values did not differ significantly among the various doses of ruxolitinib in either time point.
Table 1. Results at 1, 6, 12 months of treatment, according to different doses.
CONCLUSIONS
Ruxolitinib can effectively improve splenomegaly and alleviate constitutional symptoms in patients with myelofibrosis. The symptoms are relieved slower with the low dosage, whereas the main side effect is thrombocytopenia. It can however be maintained within safety limits (grade < 3), with dose modifications without hampering efficacy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal