Abstract
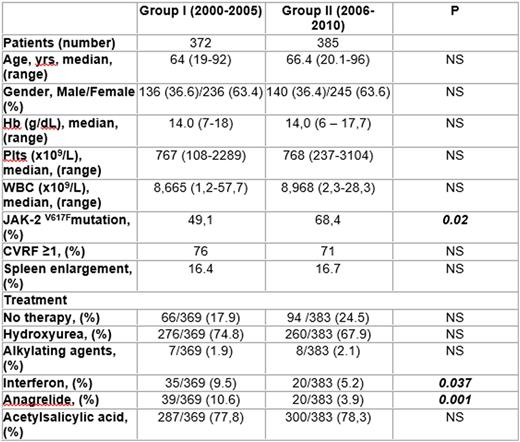
To evaluate the prognosis of patients with Essential Thrombocythemia (ET) in the first decade of the century we assessed retrospectively the thrombosis free survival (TFS) and the overall survival (OS) of the patients diagnosed from 01/01/2000 to 31/12/2009 and collected in the database of our group. The diagnosis of ET was performed with PVSG, WHO 2001 or WHO 2008 criteria, according to the period of the first observation. The whole population of 757 patients was then divided in two groups: the first (group I) with the diagnosis performed between 01/01/2000 to 31/12/2005 (334 patients) with a median follow-up of 111,9 months, the second (group II) diagnosed between 01/01/2006 to 31/12/2009 (385 patients) with a median follow-up of 58,2 months. The main clinical features of the two groups of patients are reported in the Table 1. No difference was observed between the two groups as to age, gender, platelet and WBC count, Hb level, Cardio-Vascular Risk Factors (CVRF), spleen enlargement and occurrence of previous thrombotic events. The frequency of the JAK-2 V617F mutation resulted significantly different (49.1% vs 68.4%) but in the group I the search of the mutation was never performed at the diagnosis. The TFS and OS were calculated from the date of diagnosis to the date of any appropriate event or to the date of last follow-up with Kaplan-Meier product limit method; the comparison of proportions and median values was computed with the Chi-squared and the Mann-Withney tests, as indicated. No significant difference emerged neither for TFS (p= 0,09, HR 1,42, 95% C.I. 0.89-2.30) nor for OS (p= 0,15, HR 1,34, 95% C.I. 0,87-2,06). We also considered the type of treatment used in the two groups to assess the potential link between the therapy and TFS or OS. No difference emerged between the two groups as to anti-aggregating treatment (mainly ASA), equally utilized in both groups [287/369, 77,8%, and 330/383, 78,3%, respectively (p = 0,95)]. As for the cyto-reductive therapy, Hydroxyurea was used in 74.8% vs 67.9% (p= 0.60) and alkylating agents in 1.9% vs 2.1% (p= 0.85), whereas Anagrelide was used in 10,6% vs 3,9% (p= 0,001) and Interferon in 9,5% vs 5,2% (p= 0,037), respectively. This more frequent use of Anagrelide and Interferon in the first group (2000-2005) did not modify TFS and OS of the patients. In conclusion, no improvement was observed in the prognosis of ET patients in the recent years: thus, new efforts to identify patients at risk and the introduction of new drugs as JAK-2 inhibitors are warranted to improve the prognosis of these patients.
Breccia:Bristol Myers Squibb: Honoraria; Pfizer: Honoraria; Novartis: Consultancy, Honoraria; Celgene: Honoraria; Ariad: Honoraria. Cimino:Celgene: Honoraria; Bristol-Mayer: Honoraria. Lo Coco:Pfizer: Consultancy; Baxalta: Consultancy; Novartis: Consultancy; Lundbeck: Honoraria, Speakers Bureau; Teva: Consultancy, Honoraria, Speakers Bureau. Latagliata:Novartis: Consultancy, Honoraria; Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Janssen: Consultancy, Honoraria; Shire: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal