Abstract
Background: Current European LeukemiaNet (ELN) recommendations (ELN 2013) endorse closely monitoring, due to risk of failure, for patients with so called late warning response (patients with complete cytogenetic response without major molecular response after 12 months of treatment). In this situation, for patients initially treated with imatinib, previous studies have shown a benefit of treatment change to nilotinib in terms of the achievement of deeper molecular responses. However, the role of treatment change to dasatinib in this group of patients have not been evaluated.
DASAPOST is the first clinical trial evaluating efficacy and safety of dasatinib in patients with late warning responses.
Methods: We are presenting results of the 18 patients enrolled in the phase II, open, multicenter DASAPOST study (NCT01802450). Main inclusion criteria were patients previously treated with imatinib with late suboptimal response by ELN2009 (CCyR without MMR after 18 months of treatment). The primary end point was rate of MMR by 6 moths after treatment change to dasatinib. Secondary end points were rate of MMR by 12 months, rate of deep molecular responses, progression free survival and safety of treatment change. Results were expressed as the proportion of patients who achieved molecular responses in the intention-to-treat (ITT) population, considering as failure if the evaluation was not performed in a specific time point. All BCR-ABL/ABL (IS) measurements were centralized in an EUTOS laboratory.
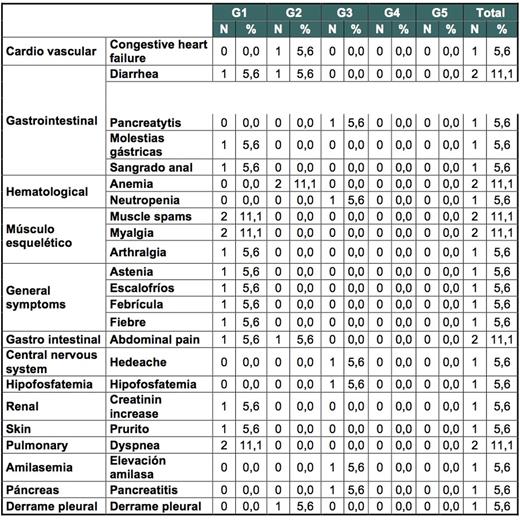
Results: From April 2013 to May 2015, 18 patients were enrolled in 12 Spanish centers. Median age was 59 years (39-77). The ratio of men to women was 13/5, and the risk groups according to Sokal Score were 48%, 30% and 22% for low, intermediate and high risk, respectively. Median time from diagnosis to treatment change to dasatinib was 2.6 years (1.6-23) and median time while on imatinib to achieve CCyR 1.4 years (0.2-12). Median exposure to imatinib was 2.4 years (1.6-14). Seventy-two percent of the patients achieved MMR by 6 months (primary endpoint). Rates of MMR and deeper molecular responses at different time points are shown in table 1. Of interest 9/18 patients (50%) achieved MR4 by 12 months. Treatment change to dasatinib was safe, with only 3 study discontinuations (16%), due to adverse events (AE) and 4 serious AE (congestive heart failure, acute gastroenteritis, pyelonephritis and pancreatitis (only congestive heart failure was related to dasatinib). Most commonly reported (>5%) drug-related AEs are shown in table 1.
Conclusions: Our study shows, for the first time, that in patients treated with imatinib and with late warning responses, switching to Dasatinib induced MMR in 2 out every 3 patients, and MR4 in half of the patients by 6 months, with a good safety profile.
Most commonly reported (>5%) drug-related AEs
García-Gutierrez:Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Casado:Ariad: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; BMS: Honoraria. Sánchez-Guijo:Pfizer: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Bristol-Myers-Squib: Consultancy, Honoraria. Martinez-Lopez:BMS: Research Funding. Steegmann:Ariad: Honoraria, Other: Research funding for the Spanish CML Group; Pfizer: Honoraria, Other: Research funding for the Spanish CML Group; BMS: Honoraria, Other: Research funding for the Spanish CML Group; Novartis: Honoraria, Other: Research funding for the Spanish CML Group.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal