Abstract
Introduction. The substitution of brand-name drugs with cheaper generics is a modern tendency in health-care to decrease the burden on government budget and improve access to efficient treatment. Worldwide, generics have to be identical to the original drug in terms of pharmaceutical (active ingredients) and biological (pharmacokinetic) properties. Unfortunately, in Russia, as in most countries, there are no government regulations of equivalency to a brand-name product regarding dosage, strength, route of administration, quality, performance, and intended use. In Russia, since August 2012 the original Imatinib has almost fully been substituted with generics for the treatment of chronic myelogenous leukemia (CML).
Aim. To assess tolerance and efficacy of Imatinib generics in terms of response durability by comparing it with that achieved previously in CML patients, who had received treatment with original Imatinib before switching to the generics.
Materials and methods. Seventy-nine CML patients treated initially with original Imatinib (Novartis AG) with median treatment duration of 6.5 years (range, 0.5-11 years) were switched to generic drugs. The drugs: 1) GenericPh 100 mg, in capsules (Ph-Syntez, Russia) - 54 patients (44 with complete cytogenetic response (CCyR), including 32 with major molecular response (MMR); 2) GenericG 100 mg, in tablets (Laboratorio TUTEUR S.A.C.I.F.I.A., Argentina) - 25 patients (22 with CCyR, including 19 with MMR). In case of loss of response, besides non-compliance, we changed treatment to second-generation tyrosine kinase inhibitors (TKI2). Switching from one generic to another was made due to intolerance. We analyzed the range and frequency of adverse events (AE) and durability of responses achieved previously (hematologic, cytogenetic and molecular). IRIS data1 was used as a comparator for AE frequency during long-term Imatinib treatment. Statistical analysis included the Fisher exact test.
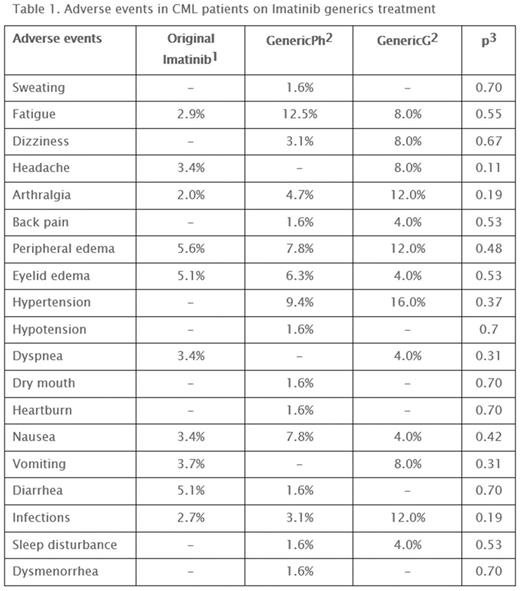
Results. Tolerance of Imatinib generics was good with few exceptions. One patient in the GenericPh group suffered severe edema and was switched to Nilotinib with AE resolution. In the GenericG group 4/25 (16%) patients had severe gastroenterological toxicity (nausea, vomiting, abdominal distension, diarrhea) and were successfully switched to GenericPh. This might have been caused by the tablet filler (lactose) and related to concomitant lactose insufficiency in these patients. One patient had frequent infectious complications. Having stable deep molecular response, she entered the treatment free remission phase and had successful molecular response for more than two years. The frequency of other AEs is presented in Table 1. No significant differences were revealed between the generics and the original Imatinib in comparison with IRIS results. However, only 25% (20) of patients were free of any AE.
No progression to AP/BC during the generics treatment was observed. Three deaths were registered (CML-related 1, blastic phase on Dasatinib treatment, the patient had only partial cytogenetic response to Imatinib and was switched to Dasatinib, with progression after 2 years on Dasatinib; cancer -1, cardiovascular disease -1). The patients who had inadequate responses to Imatinib before and after switching on generics were subsequently switched to TKI2: GenericPh - 11 patients (Nilotinib - 9 (CCyR - 8, MMR-7), Dasatinib - 2 (no CCyR with progression - 1, complete hematologic response -1)); GenericG - 3 patients (Nilotinib - 2, both with CCyR and MMR, Dasatinib - 1 -hematologic response with non-compliance). Durability of previousresponses was as follows: 4 patients lost their MMR: 3/54 (5.6%) while taking GenericPh and 1/25 (4%) - GenericG; 3 patients lost their CCyR: 2/54 (3.7%) while taking GenericPh and 1/25 (4%) - GenericG. All those patients were subsequently switched to TKI2 (3 re-achieving previous MMR and 1 demonstrating non-compliance). At the time of analysis there were still 58 (73%) patients on the generics treatment. 57 patients had CCyR and MMR, and one was not cytogenetically evaluated due to non-compliance (BCR-ABL 0.102%).
Conclusion. No significant differences between the generics studied and the original Imatinib were observed in terms of their efficacy or tolerability in CML patients who were previously treated with a brand-name drug and subsequently switched to generics.
Shuvaev:Novartis pharma: Honoraria; Pfizer: Honoraria; BMS: Honoraria. Fominykh:Novartis Pharma: Honoraria; BMS: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal