Abstract

Introduction. A generic drug is a pharmaceutical drug considered to be equivalent to a brand-name product. A generic drug has to contain the same active ingredients as those of the original formulation. Regulatory agencies used to require that generics be identical to their brand-name counterparts with regards to pharmacokinetic properties. In most cases, generic products are available after the patent protection given to a drug's original developer expires. In Russia, a patent protection lasts for a 10-year period from registration of the original drug. To this day, twelve Imatinib generics have been registered in Russia.
Aim. To assess the safety and efficacy of Imatinib generics for treatment of newly diagnosed Chronic myelogenous leukemia patients that have been in our center since August 2012.
Materials and methods. 30 newly diagnosed CML patients were started on generics. The drugs: 1) GenericPh 100 mg, in capsules (Ph-Syntez, Russia); 2) GenericG 100 mg, in tablets (Laboratorio TUTEUR S.A.C.I.F.I.A., Argentina); 3) GenericIm 100 mg, in tablets (Sandoz d.d. (Slovenia). Switching from one generic to another was done due to intolerance. We analyzed the range and frequencies of adverse events (AE), cumulative incidences of complete hematologic (CHR), major cytogenetic (MCyR), complete cytogenetic (CCyR), and early molecular responses (BCR-ABL<10% by IS), as well as the rate of BCR-ABL<1% by IS, major molecular (MMR) and molecular 4.0 log (MR4.0, BCR-ABL<0.01% by IS) responses at time-points according to the National CML diagnostic and treatment guidelines. The response rates were assessed only in regard to the generic treatment (with death, progression and switching to second-generation inhibitors as competing risks). Statistical analysis included descriptive statistics and cumulative incidence function.
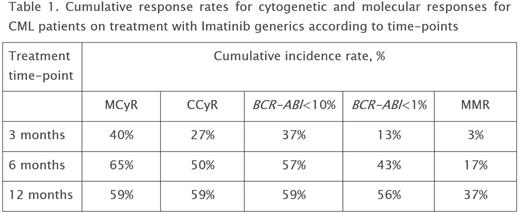
Results. Duration of the treatment with generics was 7-45 months, with a median of 13 months (GenericPh (27) + GenericG (2) + GenericIm (1)). No unexpected adverse events were observed during the Imatinib generics treatment. The generics tolerance did not differ from that of the original brand-name drug. Six patients were switched to second-generation tyrosine kinase inhibitors (TKI2) due to Imatinib intolerance. One patient progressed to blastic phase at 3 months after diagnosis. Three deaths were registered (1 - due to CML and 2 due to concomitant diseases). Overall survival rate was 90% and CML-related mortality - 3%. CHR at 3 months of the treatment was achieved in 93% of the patients. Cumulative response rates for cytogenetic and molecular responses are presented in Table 1. MR4.0 was registered in 23% of patients during overall treatment.
Seven patients were switched to TKI2 due to insufficient efficacy of Imatinib. At the time of analysis 13 patients remained on Imatinib generics treatment: 12 patients with CCyR and 1 with PCyR, including 10 patients with MMR.
Conclusion. Use of generics demands evaluation of its equivalency and control during its adoption into clinical practice. In terms of efficacy or tolerance no significant differences between the Imatinib generics studied and the original brand-name drug in newly diagnosed CML patients were found.
Shuvaev:Pfizer: Honoraria; BMS: Honoraria; Novartis pharma: Honoraria. Fominykh:BMS: Honoraria; Novartis Pharma: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal