Abstract
Background: Both the simplified Pulmonary Embolism Severity Index (sPESI) and the multivariable In-hospital Mortality for Pulmonary embolism using Claims daTa (IMPACT) rule classify patients' risk of early post-pulmonary embolism (PE) complications.
Objective: To externally validate sPESI and IMPACT for predicting 90-day all-cause mortality and readmission rates among PE patients treated within the Veterans Health Administration (VHA).
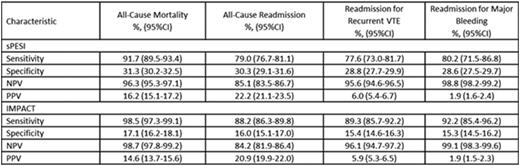
Methods: We used VHA data from 10/1/2010-9/30/2015 to identify adult patients with: (1) ≥1 inpatient diagnosis for acute PE (International Classification of Diseases-9th Revision-Clinical Modification codes=415.1x), (2) continuous medical and pharmacy enrollment for ≥12-months prior to the index PE (baseline period), (3) a minimum of 90-days of post-event follow-up or until death (whichever came first), and (4) ≥1 claim for an anticoagulant during the index PE stay. Patients were excluded if they had a claim for PE or an anticoagulant during the baseline period. We classified patients as low-risk for early post-PE complications if their sPESI score=0 or their absolute in-hospital mortality risk estimated by IMPACT was <1.5% (the latter calculated using the formula: 1/(1 + exp(-x); where x = −5.833 + [0.026*age] + [0.402*myocardial infarction] + [0.368*chronic lung disease] + [0.464*stroke] + [0.638*prior major bleeding] + [0.298*atrial fibrillation] + [1.06 1*cognitive impairment] + [0.554*heart failure] + [0.364*renal failure] + [0.484*liver disease] + [0.523*coagulopathy] + [1.068*cancer]). Sensitivity, specificity, negative and positive predictive value (NPV and PPV) for all-cause mortality, all-cause readmission, and readmission for recurrent venous thromboembolism (VTE) or major bleeding at 90-days were reported with 95% confidence intervals (CIs) for sPESI and IMPACT tools.
Results: Of6,746 eligible PE patients, 851 (12.6%) died and 1,359 (20.1%) were readmitted for any reason within 90-days. Hospitalization for recurrent VTE and major bleeding occurred in 375 (5.6%) and 116 (1.7%), respectively.sPESI classified 1,918 (28.4%) as low-risk, while 1,024 (15.2%) were low-risk per IMPACT. Both tools displayed sensitivity >90% and NPVs >96% for all-cause 90-day mortality, but low specificity and PPVs (Table). IMPACT's sensitivity for all-cause readmission was numerically higher than sPESI, but both had comparable NPVs. Similar trends were observed for accuracy in predicting readmissions due to recurrent VTE or major bleeding.
Conclusion: In this external validation study utilizing VHA data, IMPACT classified patients for 90-day post-PE outcomes with similar accuracy as sPESI. While not recommended for prospective clinical decision-making, IMPACT appears useful for identification of PE patients at low-risk for early mortality or readmission in retrospective claims-based studies.
Test characteristics for sPESI and IMPACT for 90-day post-pulmonary embolism outcomes CI= confidence interval; IMPACT=In-hospital Mortality for Pulmonary embolism using Claims data; NPV=negative predictive value; PPV=positive predictive value; sPESI=simplified Pulmonary Embolism Severity Index; VTE=venous thromboembolism
Test characteristics for sPESI and IMPACT for 90-day post-pulmonary embolism outcomes CI= confidence interval; IMPACT=In-hospital Mortality for Pulmonary embolism using Claims data; NPV=negative predictive value; PPV=positive predictive value; sPESI=simplified Pulmonary Embolism Severity Index; VTE=venous thromboembolism
Kumar:Johnson & Johnson: Employment. Wells:Itreas: Other: Served on a Writing Committee; Janssen Pharmaceuticals: Consultancy; Bayer Healthcare: Other: Speaker Fees and Advisory Board; BMS/Pfizer: Research Funding. Peacock:Comprehensive Research Associates LLC: Equity Ownership; Cardiorentis: Consultancy, Research Funding; The Medicine's Company: Consultancy, Research Funding; Banyan: Research Funding; Emergencies in Medicine LLC: Equity Ownership; Abbott: Research Funding; Alere: Consultancy, Research Funding; Prevencio: Consultancy; Janssen: Consultancy, Research Funding; Portola: Consultancy, Research Funding; Pfizer: Research Funding; Roche: Research Funding; ZS Pharma: Consultancy, Research Funding; Ischemia Care: Consultancy; Phillips: Consultancy. Fermann:Janssen Pharmaceuticals: Other: Advisory Board, Speakers Bureau; Pfizer: Research Funding. Wang:Janssen Pharmaceuticals: Research Funding. Baser:Janssen Pharmaceuticals: Research Funding. Schein:Johnson & Johnson: Employment, Equity Ownership, Other: Own in excess of $10,000 of J&J stock. Crivera:Johnson & Johnson: Employment, Equity Ownership, Other: Owns excess of $10,000 in stock. Coleman:Boehringer-Ingelheim Pharmaceuticals, inc.: Consultancy, Research Funding; Bayer Pharmaceuticals AG: Consultancy, Research Funding; Janssen Pharmaceuticals: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal