Abstract
Introduction:
Ibritumomab tiuxetan (Zevalin¨) is a radioimmunoconjugate agent that targets CD20-expressing B cell lymphomas. Currently, it is the only radioimmunoconjugate agent available on the market and is FDA approved for relapsed/refractory follicular lymphoma (FL). A retrospective study of patients with low grade FL (WHO grade 1 and 2) treated with ibritumomab tiuxetan at Mayo Clinic Florida from 2000 to 2016 was performed.
Methods:
The patient population consisted of previously untreated as well as relapsed low grade FL. We evaluated patient characteristics, response rate, duration of response, progression free survival, overall survival and time to next treatment. Previously untreated patients were treated with ibritumomab tiuxetan due to ineligibility for standard chemotherapy and prolonged immunotherapy, or their preference. Comparison between previously untreated FL patients treated with ibritumomab tiuxetan as the first line of treatment versus patients with relapsed FL treated with ibritumomab tiuxetan as second, third or fourth line therapy was made.
Results:
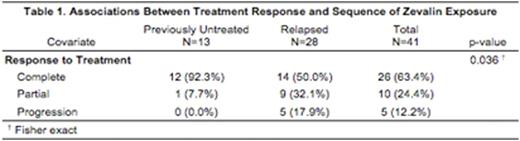
Low grade FL patients (n=41) that received ibritumomab tiuxetan were identified; 13 patients (32%) with previously untreated FL and 28 patients (68%) with relapsed FL. The data on response to treatment is presented in Table 1. Ibritumomab tiuxetan use in previously untreated FL was associated with more complete responses than in the relapse setting (92% vs 50% at 6 months, p=0.036).
Previously untreated FL patients that received ibritumomab tiuxetan as first line therapy had better progression free survival (80% vs 35% at 3 years). Ibritumomab tiuxetan use in relapsed FL was associated with significant shorter time to relapse compared to previously untreated FL (median 1.3 years vs 4.8 years, p=0.017, figure 1) and shorter time to next treatment (65% at 4 years compared to 0% in previously untreated, p=0.007). 15% of newly diagnosed FL treated with ibritumomab tiuxetan relapsed compared to 64% of relapsed FL patient. There was no significant difference in overall survival between the two groups.
The incidence of grades 3-4 neutropenia (Absolute Neutrophil Count <1000 x109/L), Anemia (Hb <8 g/dL) and Thrombocytopenia (Platelets <25 x109/L) were 47%, 7.7% and 48.7% respectively. Only 6/41 patients (15%) had cytopenias that required transfusion of blood products. More significant cytopenias were seen in relapsed group compared to previously untreated group.
Conclusions:
Ibritumomab tiuxetan as first line treatment in previously untreated FL patients at our institution was associated with improved response rate, better progression free survival and shorter time to next treatment when compared to its use in relapsed FL.
Ibritumomab tiuxetan has significant therapeutic efficacy for both previously untreated as well as relapsed low grade FL. It was well tolerated without significant treatment-related morbidity or mortality. Moreover, treatment schedule was very convenient for the patients.
Our results suggest that treatment with ibritumomab tiuxetan is an effective option for those previously untreated low grade FL patients who cannot take standard chemoimmunotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal